Colors the flame a bright yellow-orange color. Metals of the main subgroups
Flames come in different colors. Look into the fireplace. Yellow, orange, red, white and blue flames dance on the logs. Its color depends on the combustion temperature and the combustible material. To visualize this, imagine the spiral of an electric stove. If the tile is turned off, the spiral turns are cold and black. Let's say you decide to heat up the soup and turn on the stove. At first the spiral turns dark red. The higher the temperature rises, the brighter the red color of the spiral. When the tile warms up to maximum temperature, the spiral turns orange-red.
Naturally, the spiral does not burn. You don't see the flame. She's just really hot. If you heat it further, the color will change. First, the color of the spiral will turn yellow, then white, and when it heats up even more, a blue glow will emanate from it.
What determines the color of the flame
Something similar happens with fire. Let's take a candle as an example. Different sections of a candle flame have different temperatures. Fire needs oxygen. If you cover the candle glass jar, the fire will go out. The central area of the candle flame adjacent to the wick consumes little oxygen and appears dark. The top and side areas of the flame receive more oxygen, so these areas are brighter.
As the flame moves through the wick, the wax melts and crackles, breaking into tiny carbon particles. (Coal also consists of carbon.) These particles are carried upward by the flame and burn. They are very hot and glow like the spiral of your tile. But the carbon particles are much hotter than the coil of the hottest tile (carbon combustion temperature is approximately 1,400 degrees Celsius). Therefore, their glow has yellow. Near the burning wick, the flame is even hotter and glows blue.
The flames of a fireplace or fire are mostly variegated. Wood burns at a lower temperature than a candle wick, so the base color of the fire is orange rather than yellow. Some carbon particles in a fire flame have a fairly high temperature. There are few of them, but they add a yellowish tint to the flame. Cooled particles of hot carbon are soot that settles on the chimneys. The burning temperature of wood is lower than the burning temperature of a candle. Calcium, sodium and copper, when heated to high temperatures, glow in different colors. They are added to rocket powder to color the lights of holiday fireworks.
Flame color and chemical composition
The color of the flame may vary depending on the chemical impurities contained in the logs or other flammable substance. The flame may contain, for example, sodium impurities. Sodium is a component table salt. When sodium is heated, it turns bright yellow. Calcium may be released into the fire.
We all know that milk contains a lot of calcium. It's metal. Hot calcium turns bright red. If phosphorus burns in a fire, the flame will turn greenish. All these elements are either contained in wood or enter the fire with other substances. Mixing the colors of a flame, like mixing the colors of a rainbow, can give White color, which is why white areas are visible in the flame of a fire or fireplace.
GD Star Rating
a WordPress rating system
Laboratory work 1-2
Qualitative analysis of cations
Na+
Flame color reaction
Dip a clean hot wire into a solution of sodium chloride or put a little solid salt on it. Introduce the wire along with droplets or particles of sodium salt into the colorless flame of the burner - the flame will turn yellow.
K+
Flame color reaction
Volatile potassium compounds color the colorless flame with its characteristic purple. The violet color of the flame in the presence of sodium salts becomes invisible, since sodium compounds color the burner flame yellow.
Reactions with sodium hexanitrocobaltate (ΙΙΙ)

Place 1-2 drops of a solution of any potassium salt in a test tube, add 3-5 drops of sodium hexanitrocobaltate (ΙΙΙ) solution to it, add a few drops of 6 M acetic acid and rub the glass rod against the sides of the test tube. In this case it will fall out yellow crystalline precipitate dipotassium sodium hexanitrocobaltate (ΙΙΙ) 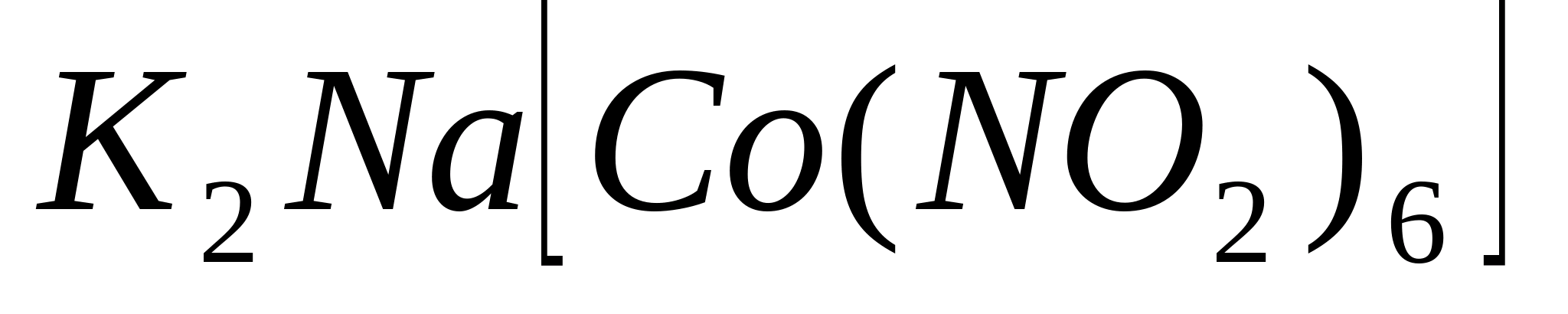 :
:
or in ionic form:
The reaction should be carried out in the presence of dilute acetic acid.
Reaction with sodium hydrogen tartrate

Place 2-3 drops of a solution of any potassium salt into a test tube, add 0.5 ml of sodium hydrogen tartrate solution and rub the glass rod against the walls of the test tube. After some time, a white crystalline precipitate will form:
or in ionic form:
Reaction conditions.
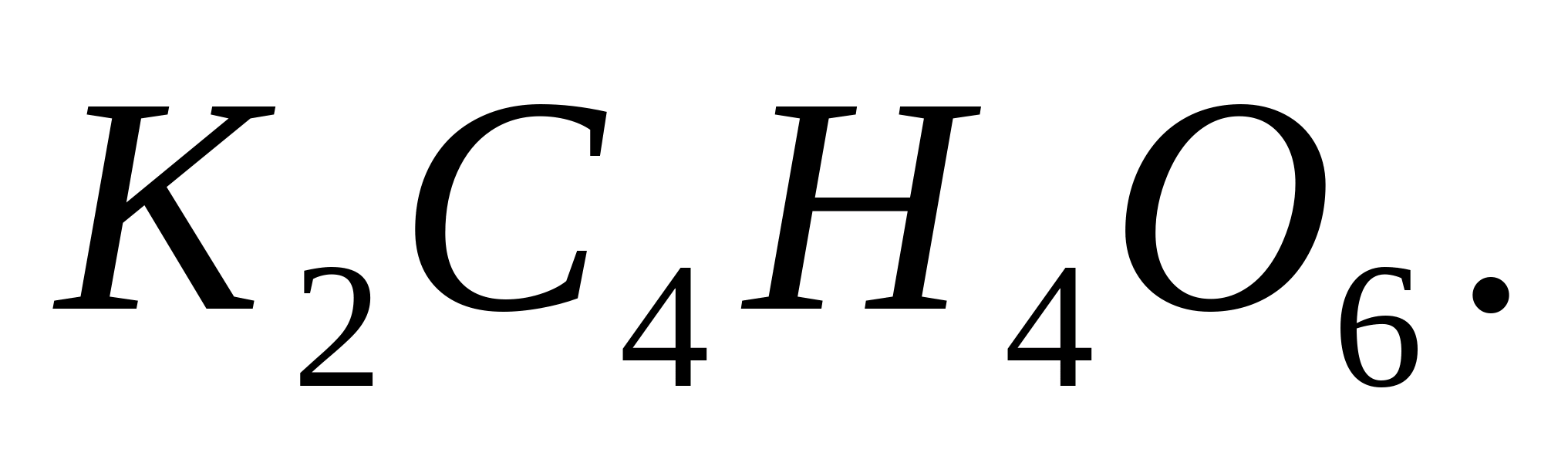
NH4+
Reaction with Nessler's reagent

Add to drop
dilute ammonium salt solution 1-2 drops of reagent solution. In the presence  - ions form a characteristic red-brown precipitate; if traces are present, the solution turns yellow:
- ions form a characteristic red-brown precipitate; if traces are present, the solution turns yellow:
or in ionic form:
Other cations of the I analytical group do not interfere with the detection of ammonium ions by the Nessler reagent.
Reaction with alkalis
Place a few drops of ammonium salt solution in a test tube  and add 5 drops of an aqueous solution of any of the strong bases -
and add 5 drops of an aqueous solution of any of the strong bases -  - and heat the contents of the test tube in the flame gas burner. Due to the decomposition of ammonium salt ammonia will be released:
- and heat the contents of the test tube in the flame gas burner. Due to the decomposition of ammonium salt ammonia will be released:
or in ionic form:
Ammonia released can be detected in various ways:
by smell;
by the blueness of universal indicator paper moistened with distilled water and added to the vapor above the solution;
by the formation of ammonium chloride smoke when a glass rod moistened with a drop of concentrated hydrochloric (hydrochloric) acid is brought to the opening of the test tube.
Boiling with caustic alkalis or sodium or potassium carbonates
When exposed to caustic alkalis or sodium or potassium carbonates, as well as during prolonged heating, ammonium salts in solutions decompose with the release of ammonia gas.
Mg++
Action of strong bases.
When strong bases are added to solutions of magnesium salts, white precipitate
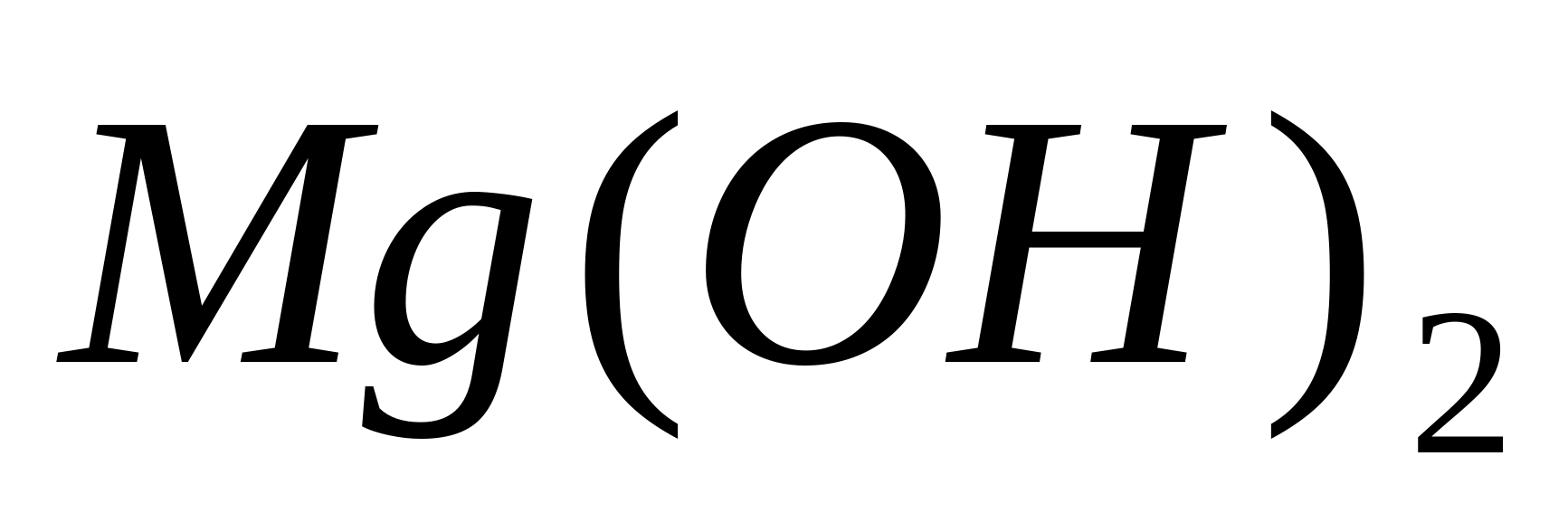 . When a weak base (ammonium hydroxide) is added, precipitation is incomplete, and in the presence of ammonium salts, no precipitate forms at all. Therefore, ammonium salts must first be removed from the solution.
. When a weak base (ammonium hydroxide) is added, precipitation is incomplete, and in the presence of ammonium salts, no precipitate forms at all. Therefore, ammonium salts must first be removed from the solution.
Place a few drops of the solution in a test tube MgCl 2
, add a few drops of an aqueous solution of any of the strong bases -. Precipitation will form Mg(HE) 2
.
Into another test tube to the solution MgCl 2
instead of  add ammonium hydroxide solution. Note which tube produces more sediment.
add ammonium hydroxide solution. Note which tube produces more sediment.
Reaction with sodium monohydrogen phosphate

Microcrystalloscopic reaction.
D ![]() For microcrystalscopic detection
For microcrystalscopic detection 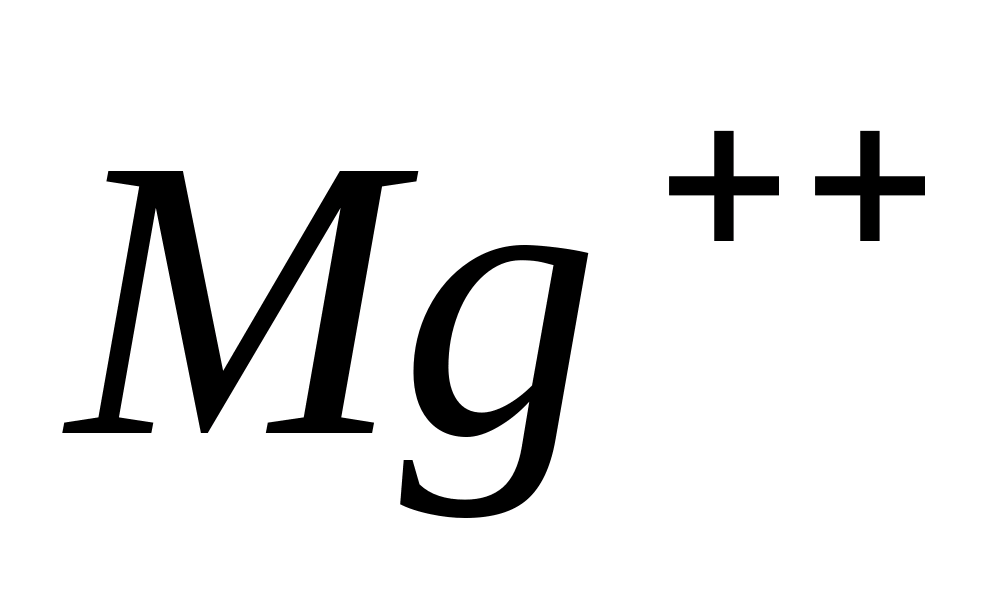 - ions in the form
- ions in the form  place a drop of solution
place a drop of solution  on slide
. Then add a drop of solution from a capillary pipette to it first
on slide
. Then add a drop of solution from a capillary pipette to it first 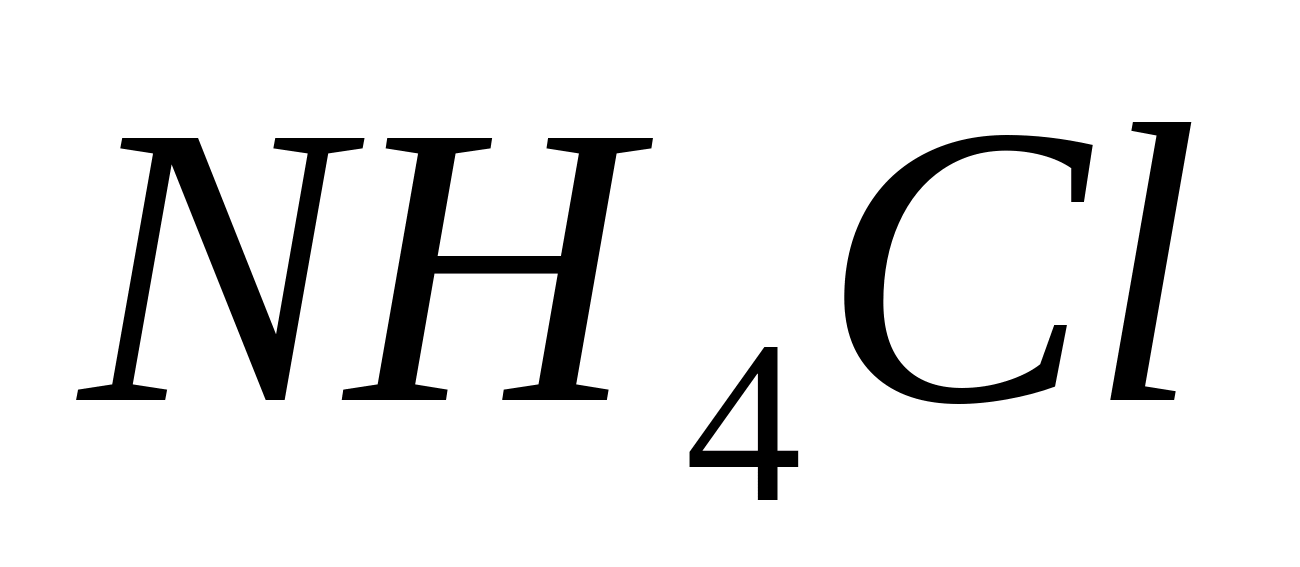 , then a drop of concentrated aqueous ammonia solution. Finally, add a crystal to the solution
, then a drop of concentrated aqueous ammonia solution. Finally, add a crystal to the solution 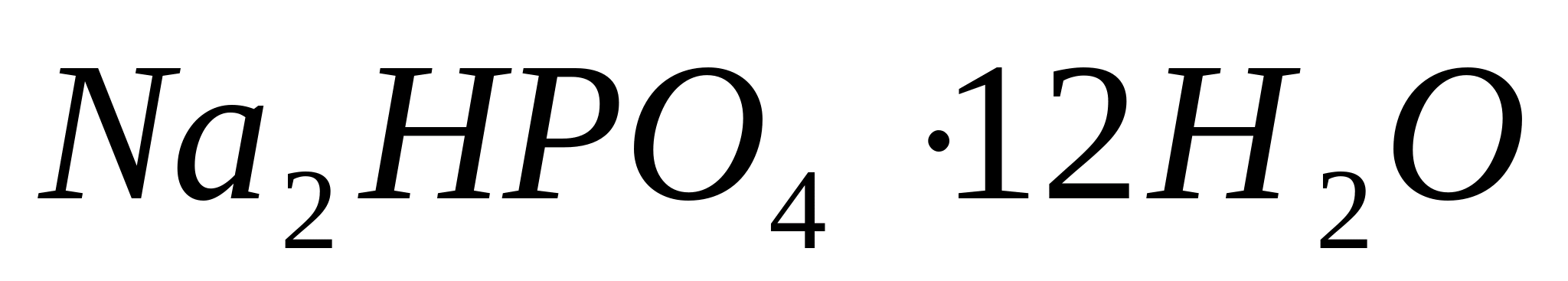 (sodium hydrogen phosphate). It is recommended to heat the slide. In the immediate vicinity of the sodium phosphate crystal, dendritic crystals appear; at a further distance, regularly formed crystals in the form of six-rayed stars appear. - magnesium ammonium phosphate
(sodium hydrogen phosphate). It is recommended to heat the slide. In the immediate vicinity of the sodium phosphate crystal, dendritic crystals appear; at a further distance, regularly formed crystals in the form of six-rayed stars appear. - magnesium ammonium phosphate 
or in ionic form
Examine the crystals under a microscope.
Ca++
Reaction with ammonium oxalate.
Place 1-2 drops of a solution of any calcium salt into a test tube, for example 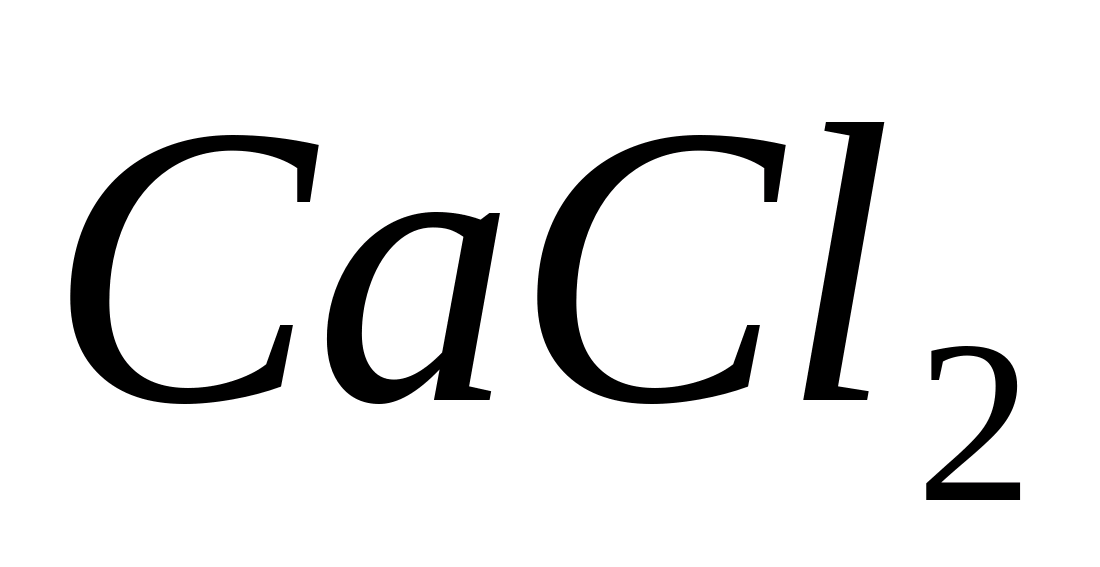 , and add 1-2 drops of acetic acid so that the reaction of the medium is acidic (in the case of the methyl red indicator, the color should turn orange). Add a few drops of ammonium oxalate solution
, and add 1-2 drops of acetic acid so that the reaction of the medium is acidic (in the case of the methyl red indicator, the color should turn orange). Add a few drops of ammonium oxalate solution 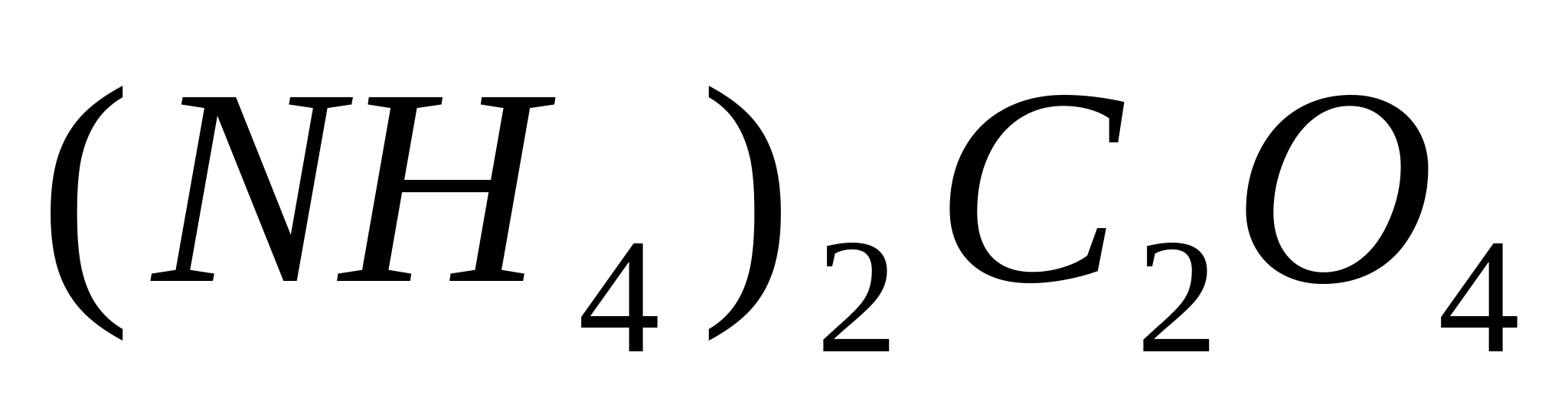 In this case, it drops out immediately from a concentrated solution, and gradually drops out from a diluted solution. white fine crystalline precipitate
In this case, it drops out immediately from a concentrated solution, and gradually drops out from a diluted solution. white fine crystalline precipitate
 . In the presence
. In the presence  Calcium oxalate is precipitated quantitatively:
Calcium oxalate is precipitated quantitatively:
or in ionic form:
Microcrystalloscopic reaction with sulfuric acid .
P  place a drop of calcium chloride solution on a glass slide, then add a drop of diluted
place a drop of calcium chloride solution on a glass slide, then add a drop of diluted  and lightly evaporate the mixture. In this case, beautiful characteristic tufts of needles –
gypsum crystals
and lightly evaporate the mixture. In this case, beautiful characteristic tufts of needles –
gypsum crystals 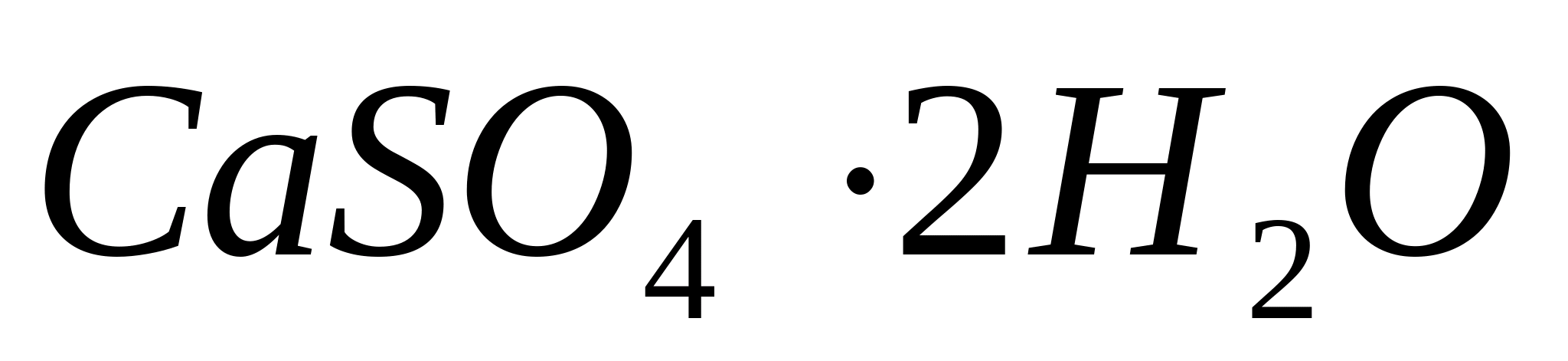 ,
easily distinguishable under a microscope.
,
easily distinguishable under a microscope.
Flame color reaction
Calcium ions color the colorless flame brick red.
Ba++
Reaction with potassium chromate (or dichromate) .
Place 1-2 drops of a solution of some barium salt into a test tube, for example  , and add a few drops of solution
, and add a few drops of solution  or
or 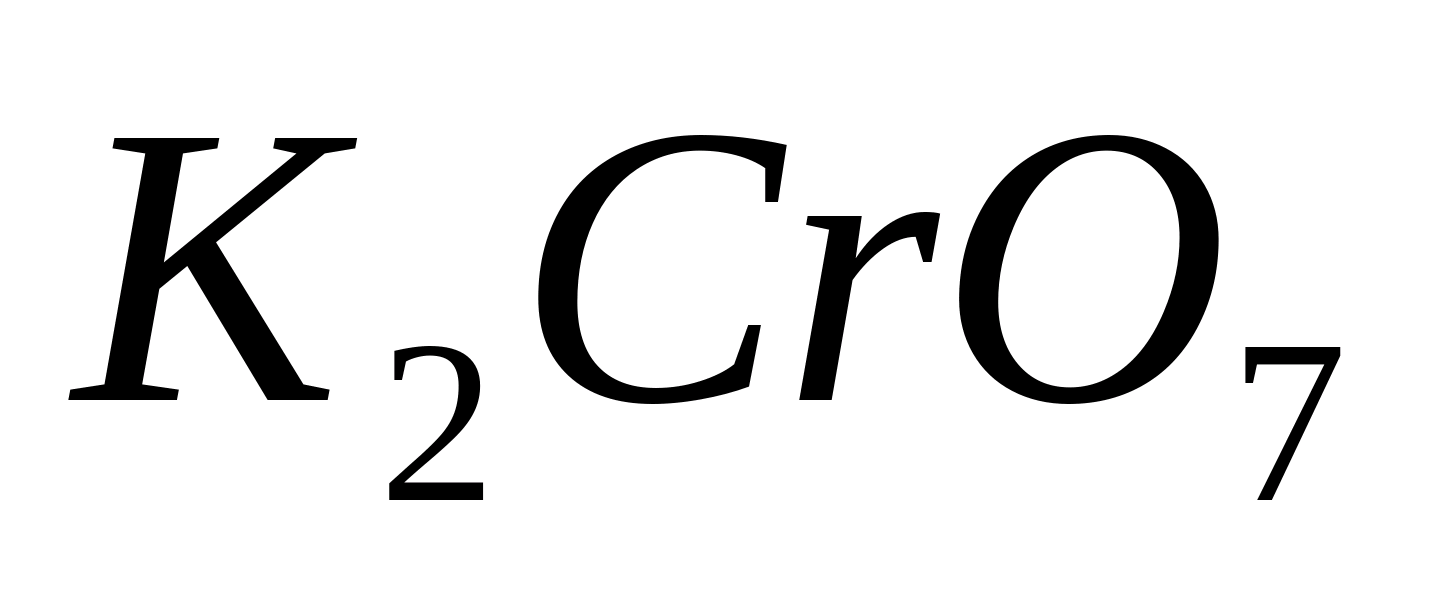 . Heat the test tube on a burner flame. In this case it falls out yellow crystalline precipitate:
. Heat the test tube on a burner flame. In this case it falls out yellow crystalline precipitate:
or in ionic form:
or in ionic form:
2![]()
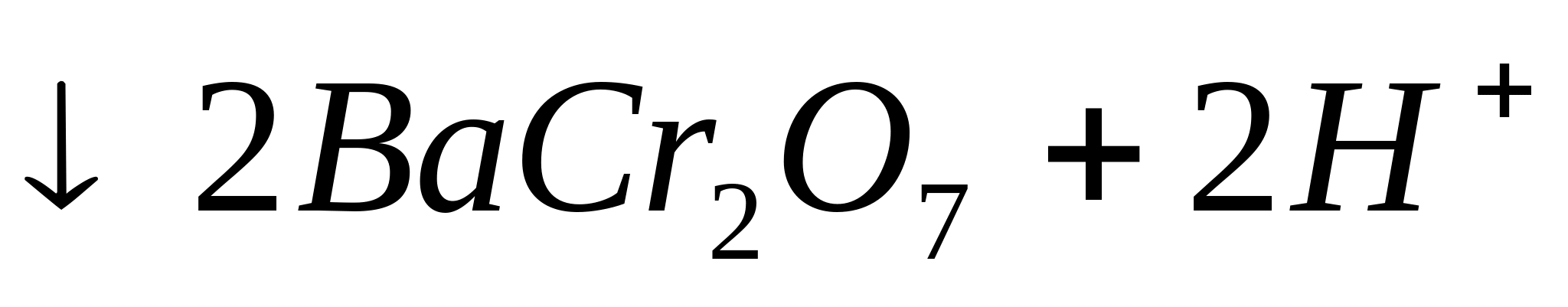
Drop reaction with sodium rhodizonate.
Place a drop of the neutral test solution and then a drop of an aqueous solution of sodium rhodizonate on the filter paper. Formed red-brown barium rhodizonate precipitate:
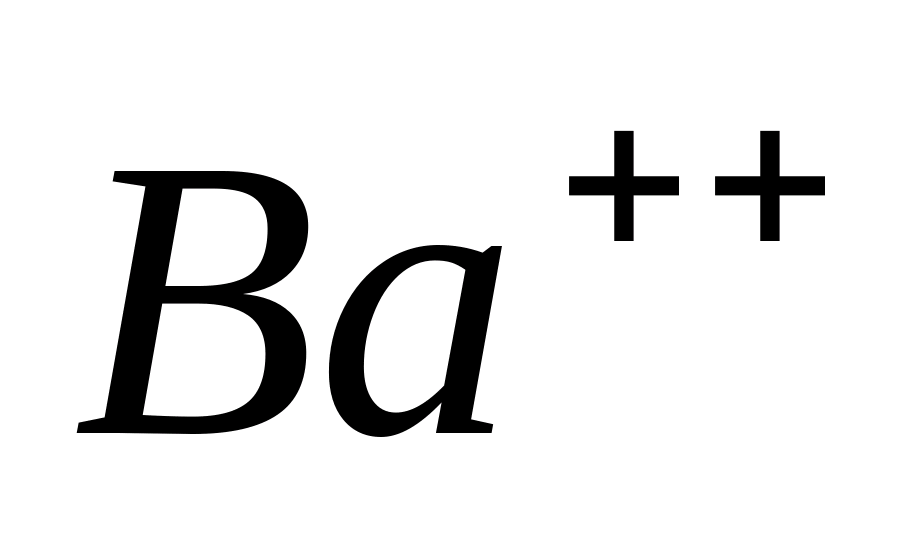 +
+
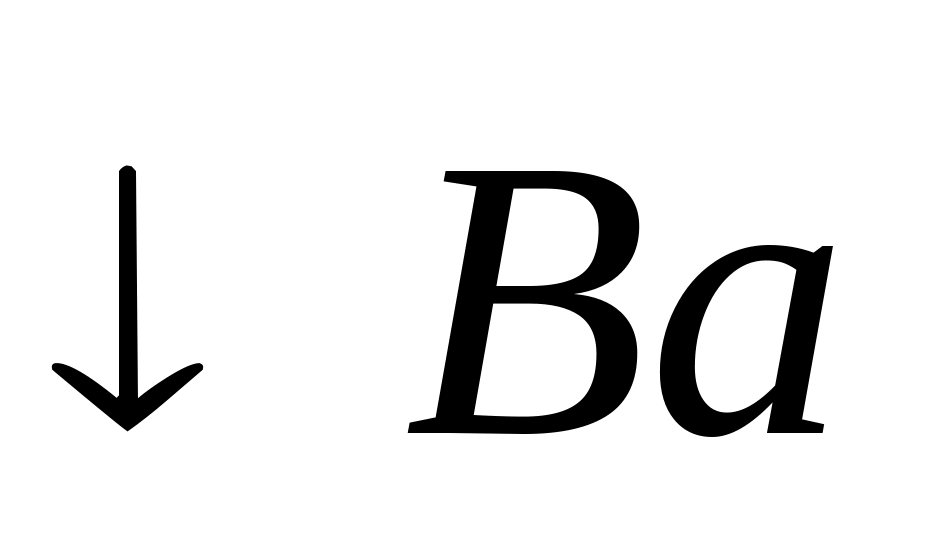 +
+
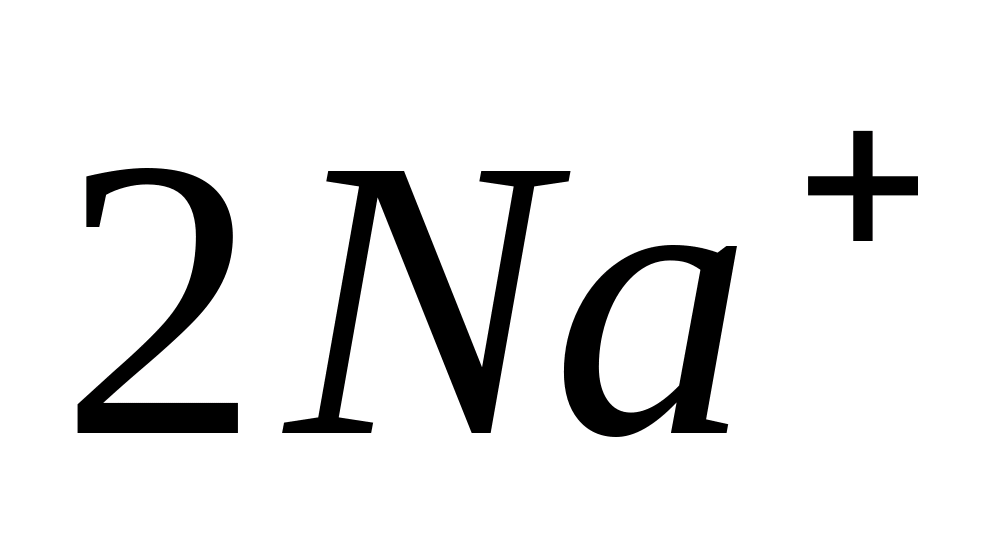
When cold in hydrochloric acid, barium rhodizonate turns into bright red barium hydrogenodizonate:
Flame color reaction.
The colorless flame is colored by barium ions yellow-green color.
Reaction with sulfuric acid or ammonium sulfate.
Place a few drops of water-soluble barium salt in a test tube, e.g.
barium chloride, add 1 ml of dilute sulfuric acid or ammonium sulfate solution. In this case it falls out white crystalline precipitate barium sulfate 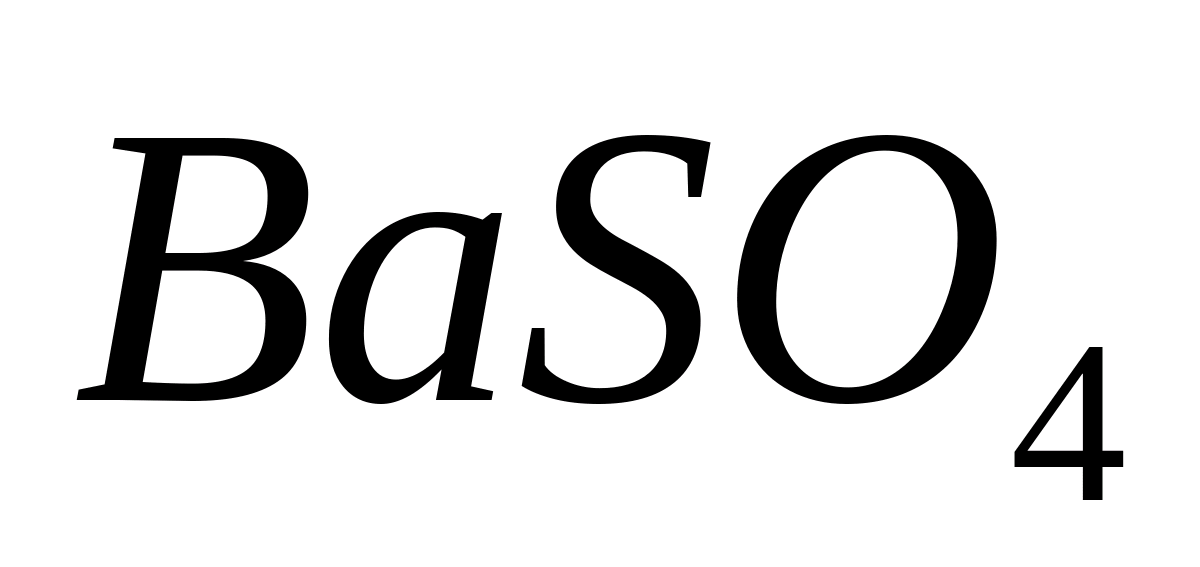 .
.
Al +++
Reaction with ammonium hydroxide.
Place 1 ml of aluminum salt solution in a test tube, e.g.  , add a few drops of ammonium hydroxide solution to it and heat it. In this case it falls out white yellowefigurativesedimenthydroxidaaluminum:
, add a few drops of ammonium hydroxide solution to it and heat it. In this case it falls out white yellowefigurativesedimenthydroxidaaluminum:
or in ionic form:
Transfer the aluminum hydroxide precipitate along with the solution into a centrifuge tube and centrifuge. Drain the clear solution and divide the precipitate into two parts.
Carry out the following test reactions:

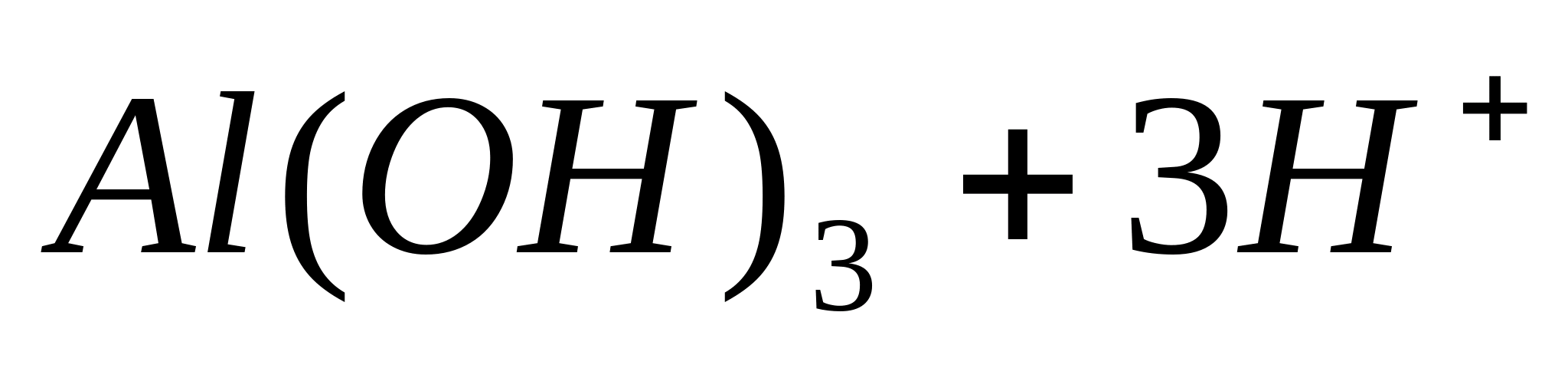

Therefore, it is a typical amphoteric compound.
Reaction with alizarin (1,2-dioxyanthraquinone)

Test tube reaction. Place 2 drops of a solution of any aluminum salt in a test tube and add 5 drops  . In this case, a precipitate forms. Add a few drops of freshly prepared alizarin solution to the resulting precipitate and boil. Alizarin forms an intensely red compound with aluminum hydroxide called aluminum varnish. Aluminum varnish does not dissolve in dilute acetic acid. Therefore, after cooling the contents of the test tube, add a little acetic acid to a slightly acidic reaction (pH ~ 4-5). In the presence of aluminum ions, the red precipitate does not disappear.
. In this case, a precipitate forms. Add a few drops of freshly prepared alizarin solution to the resulting precipitate and boil. Alizarin forms an intensely red compound with aluminum hydroxide called aluminum varnish. Aluminum varnish does not dissolve in dilute acetic acid. Therefore, after cooling the contents of the test tube, add a little acetic acid to a slightly acidic reaction (pH ~ 4-5). In the presence of aluminum ions, the red precipitate does not disappear.
Reaction conditions.
When carrying out a test tube reaction, the pH value at the beginning of precipitation should exceed 7, corresponding to a weak ammonia solution, and after precipitation, the pH may be less than 7, corresponding to a dilute acetic acid solution (pH = 4-5).
The reaction is carried out at boiling.
The presence of precipitation of other hydroxides, at least not large quantities, undesirable, and in large quantities unacceptable.
Cr+++
Oxidation C r +3 chromium in Cr +6
Place 2-3 drops of a solution of chromium (III) sulfate or nitrate in a test tube, add 5 drops of hydrogen peroxide to it  , 3-5 drops of potassium hydroxide KOH. Heat the mixture to a boil.
, 3-5 drops of potassium hydroxide KOH. Heat the mixture to a boil.
In this case, oxidation occurs  -ions up to
-ions up to 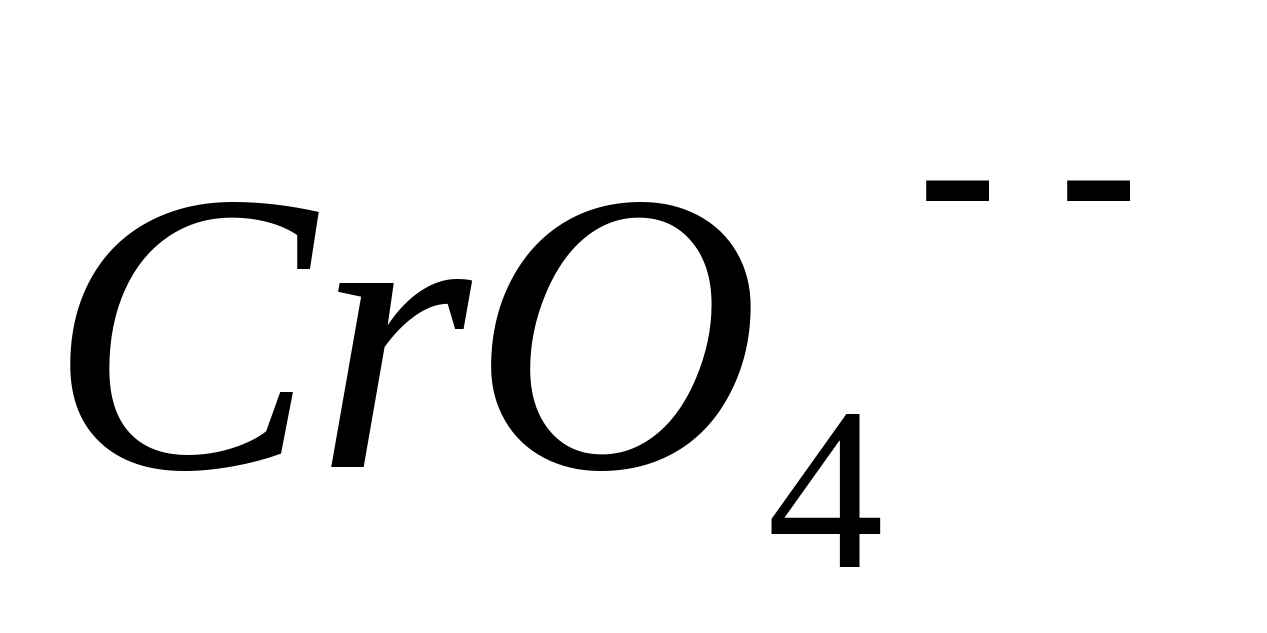 -ions and coloring from blue-green goes into yellow.
-ions and coloring from blue-green goes into yellow.
Carry out the following test reactions with the resulting solution to confirm the formation of -ions.
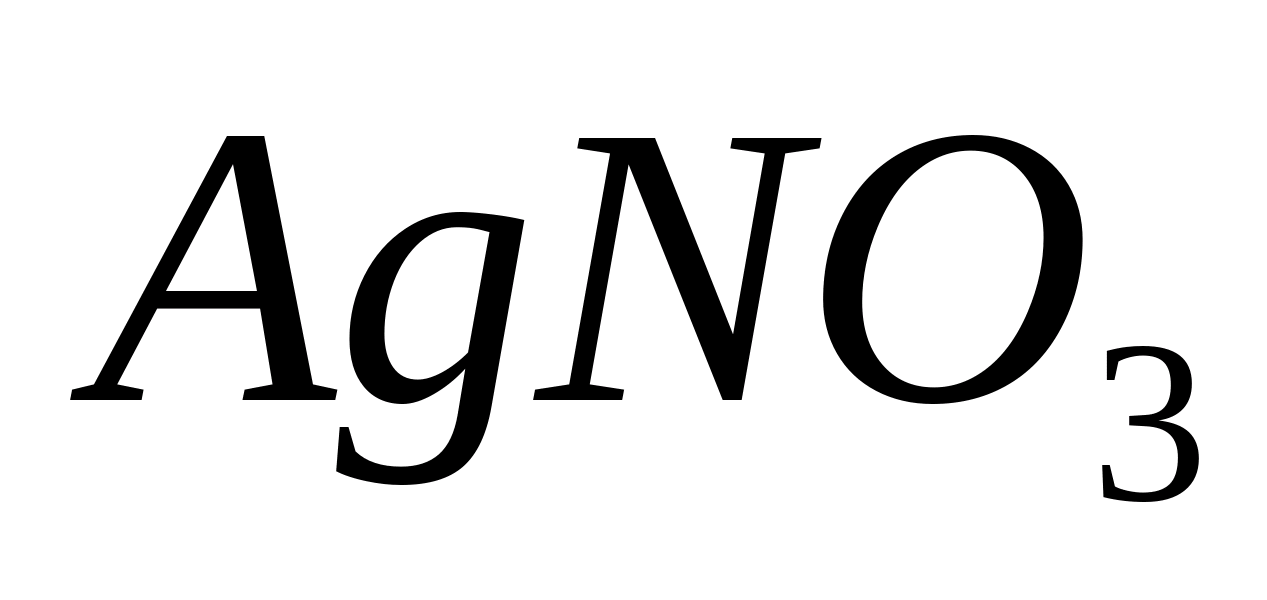
Fe +++
Reaction with 
Place 1-2 drops of solution in a test tube or on a glass slide  , acidify the solution with 1-2 drops of hydrochloric acid, add 2-3 drops of yellow blood salt - a solution of potassium hexacyanoferrate (II)
, acidify the solution with 1-2 drops of hydrochloric acid, add 2-3 drops of yellow blood salt - a solution of potassium hexacyanoferrate (II)
 In this case it falls out dark blue precipitate of Prussian blue:
In this case it falls out dark blue precipitate of Prussian blue:
Reaction with ammonium thiocyanate.
Place 1 ml of solution in a test tube, dilute it with five drops of distilled water and add 3-5 drops of ammonium thiocyanate solution
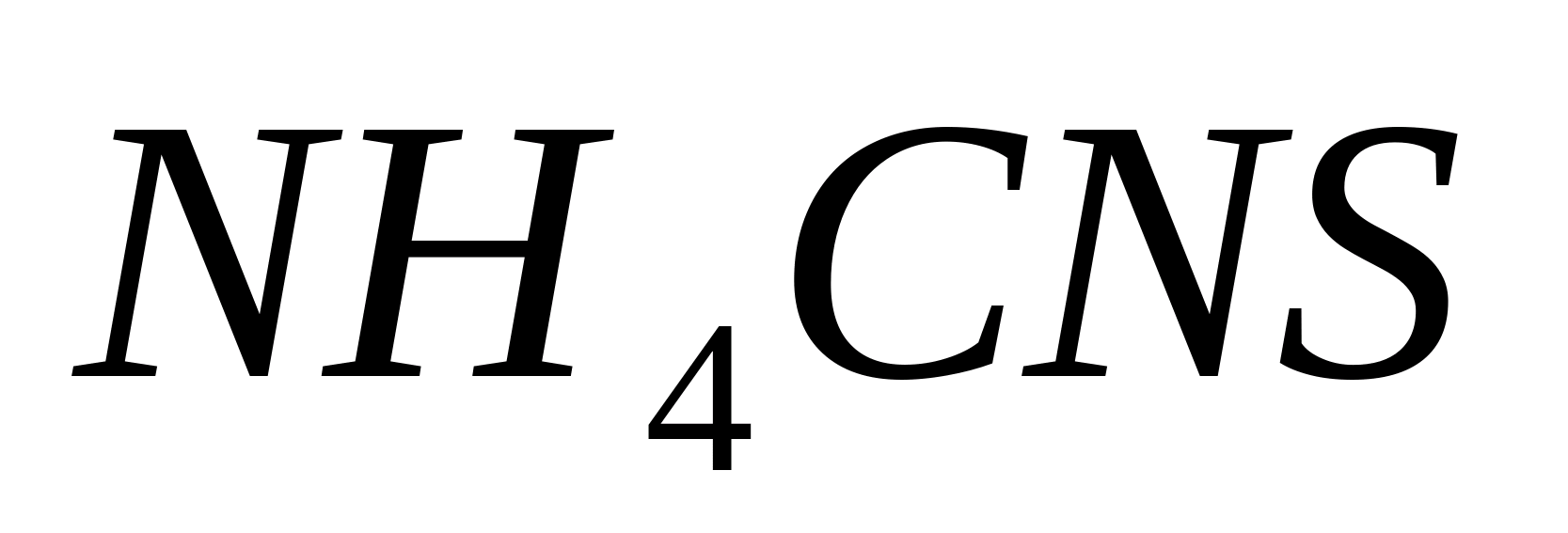 . At the same time it appears blood red coloring:
. At the same time it appears blood red coloring:
or in ionic form:
Reaction with sodium, potassium or ammonium hydroxide.
When exposed to solutions 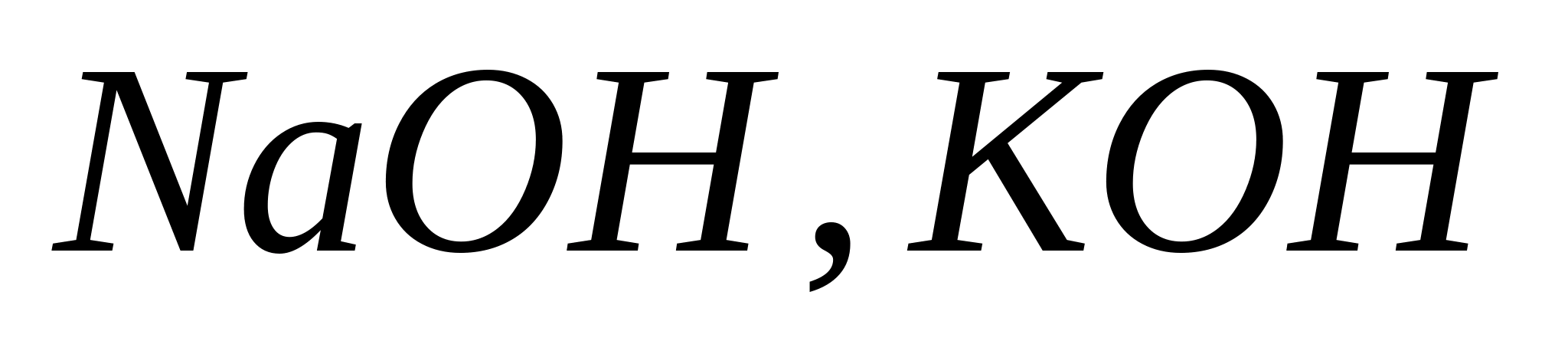 And
to ions
And
to ions 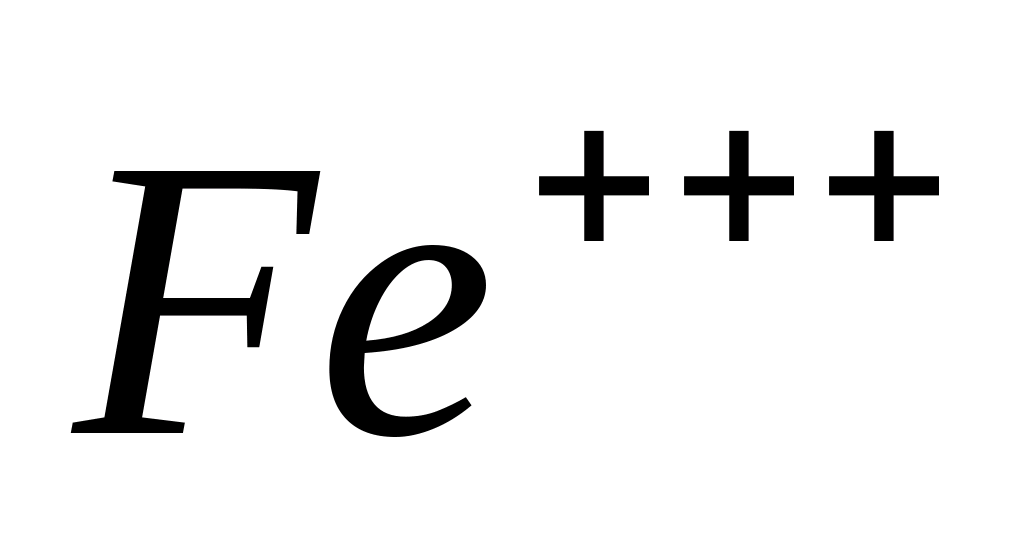 is formed brown-red sediment Fe(OH)3, soluble in acids:
is formed brown-red sediment Fe(OH)3, soluble in acids:
Fe++
Reaction with 
Place 1-2 drops of solution in a test tube or on a glass slide FeSO 4 , add 2-3 drops red blood salt - solution of potassium hexacyanoferrate (III) In this case, formation is observed turnbull blue:
Zn++
Reaction with sodium, potassium and ammonium hydroxide.
When sodium or potassium hydroxide reacts with zinc chloride, it forms white precipitate Zn(OH) 2 , soluble in excess and .
Obtain a precipitate of zinc hydroxide in a test tube and separate it from the solution using a centrifuge. Divide the sediment into two parts. Dissolve one part of the precipitate in an acid solution, the other in a base solution. Write reaction equations confirming the amphoteric nature of zinc hydroxide.
Reactions of Mn++ ions
Reaction with sodium hydroxide and hydrogen peroxide.
Manganese ions are characterized by oxidation-reduction reactions.
One of the characteristic oxidation reactions 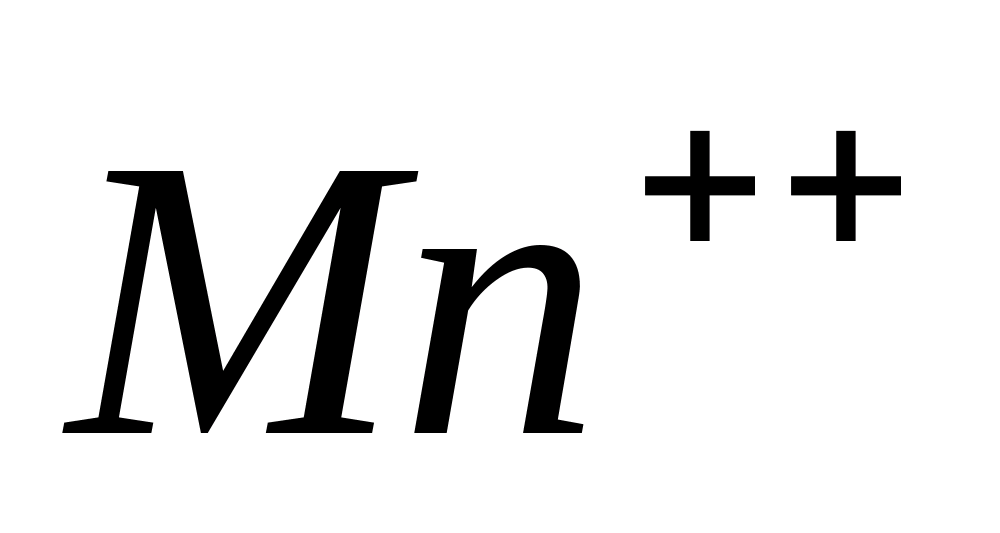 in an alkaline environment is its interaction with. When exposed to hydrogen peroxide in an alkaline environment, colorless manganese (II) ions are oxidized into insoluble manganese (IV) compounds.
in an alkaline environment is its interaction with. When exposed to hydrogen peroxide in an alkaline environment, colorless manganese (II) ions are oxidized into insoluble manganese (IV) compounds. 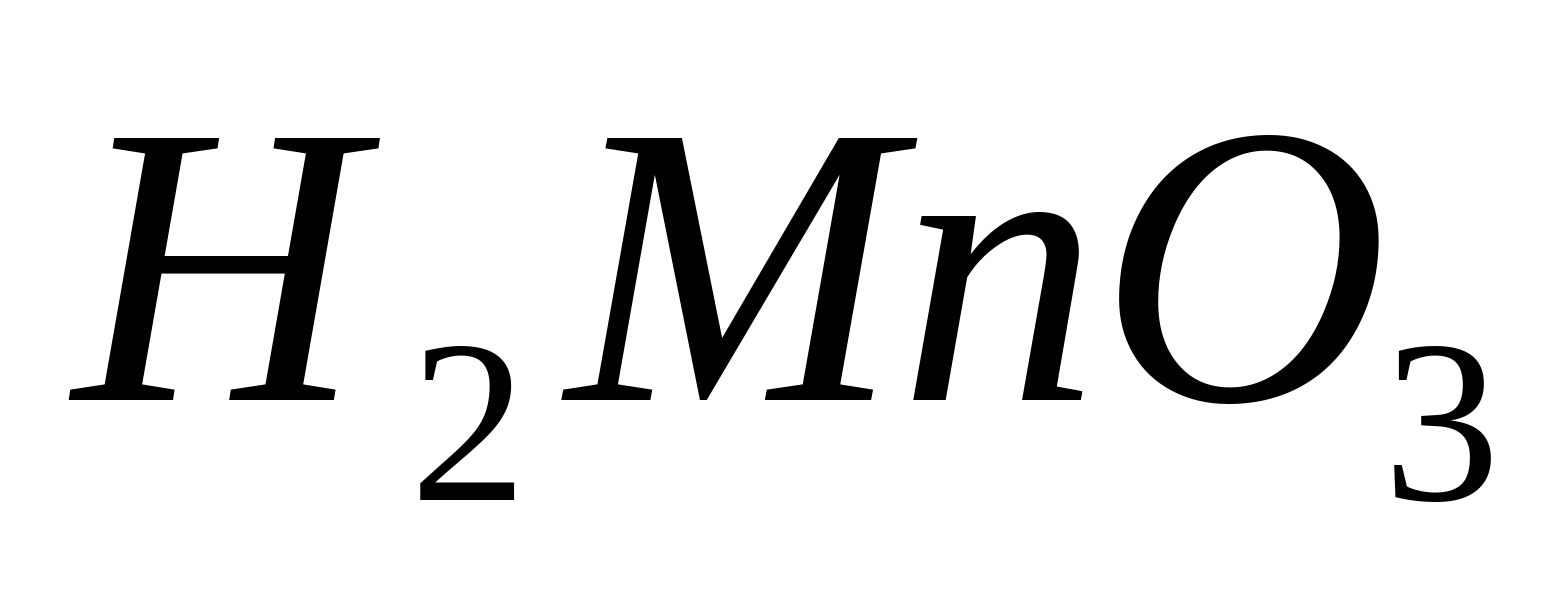 or
or 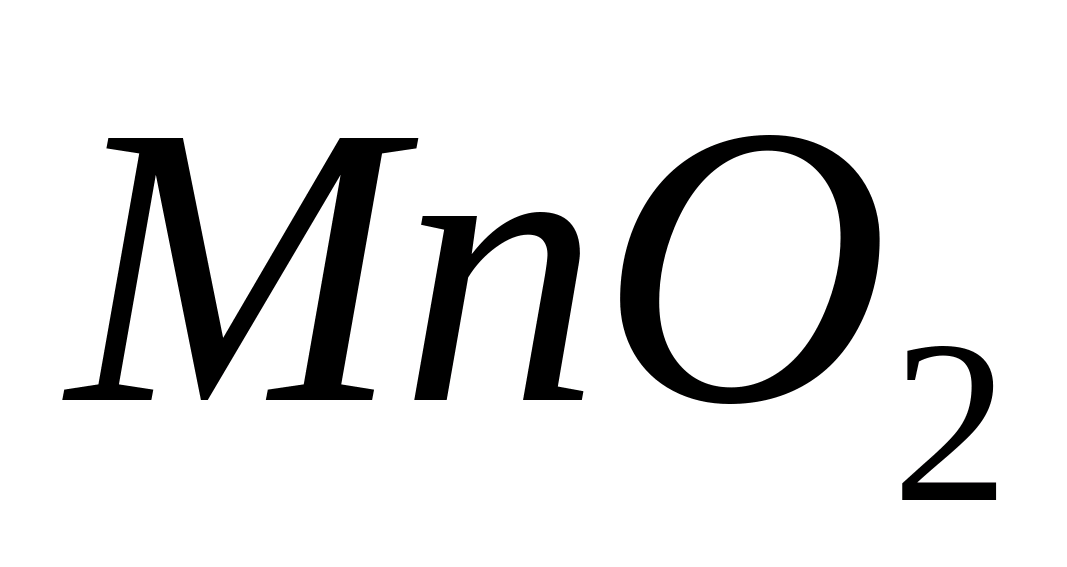 , painted brown:
, painted brown:
or in ionic form
Carry out the oxidation of -ions to. To do this, place 1-3 drops of a solution of any manganese salt into a test tube and add a few drops of NaOH solution. Formed white precipitate manganese hydroxide, slowly turning brown due to oxidation in air:
Add a few drops to the resulting precipitate. The precipitate instantly becomes brown-black due to the rapid oxidation of manganese (II) ions.
Reaction conditions.
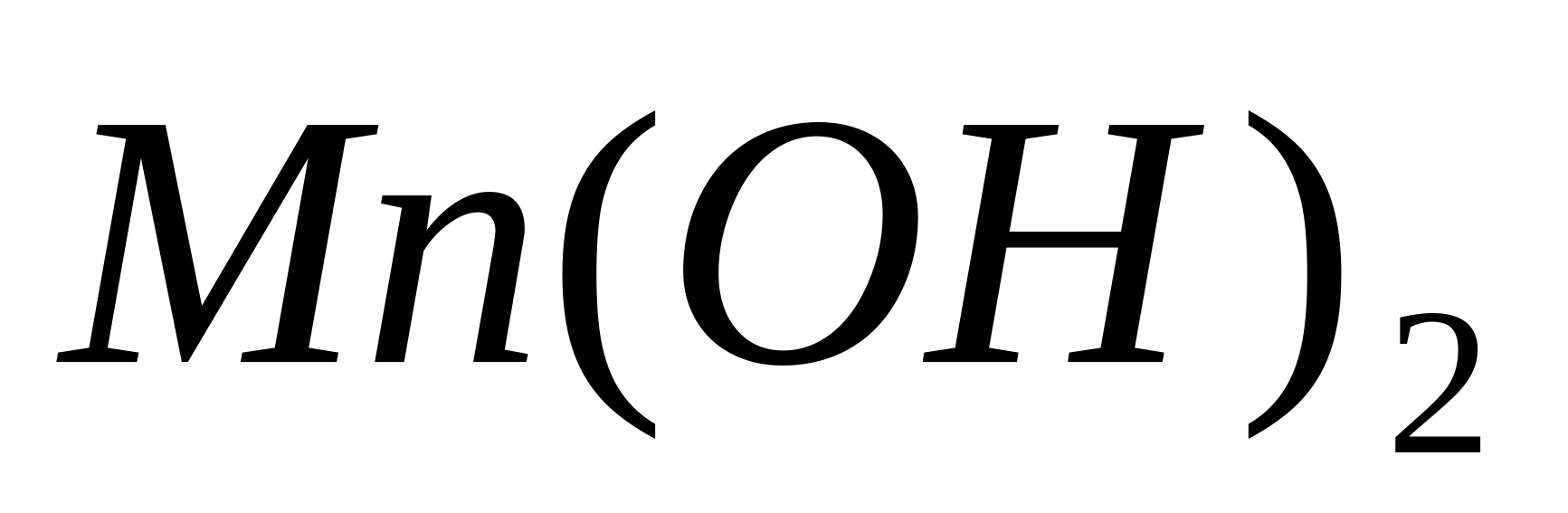
Oxidation
-ions up to
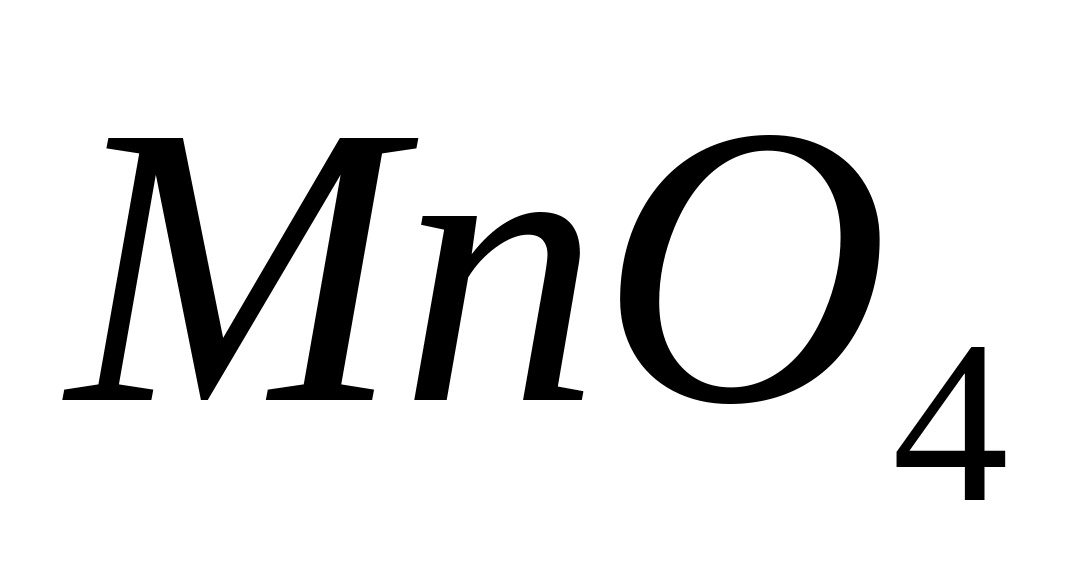 - -ions in an acidic environment.
- -ions in an acidic environment.
Manganese (II) compounds are oxidized in an acidic environment by strong oxidizing agents into permanganic acid. One of the most important oxidation reactions in a nitric acid or sulfuric acid environment is the interaction of -ions with  or
or 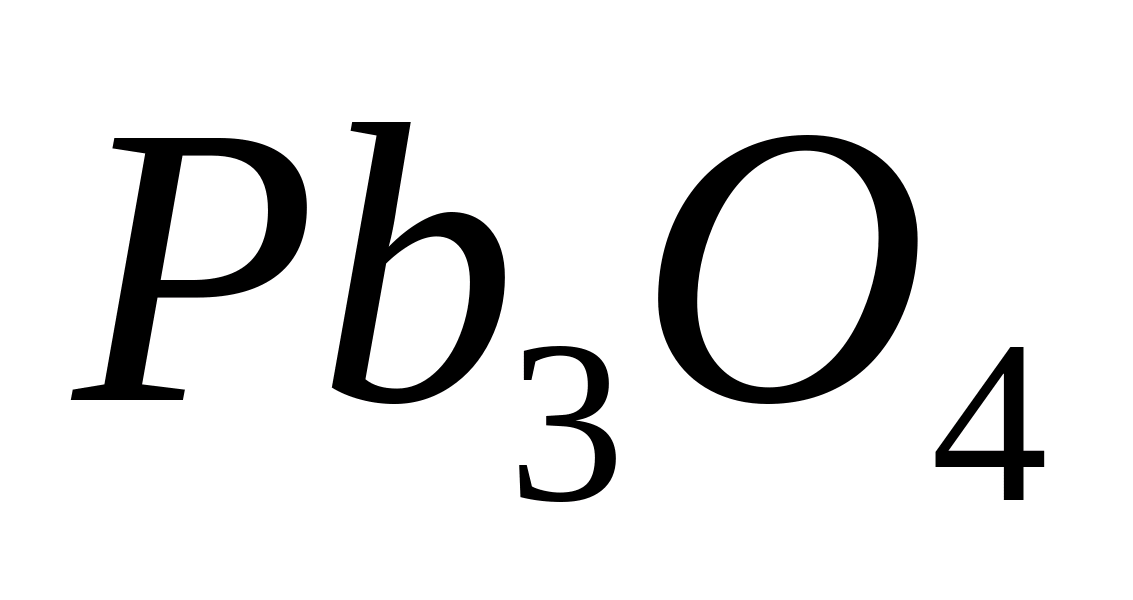 . In this case, colorless compounds of divalent manganese () are oxidized to manganese compounds with an oxidation state of +7 (
. In this case, colorless compounds of divalent manganese () are oxidized to manganese compounds with an oxidation state of +7 ( 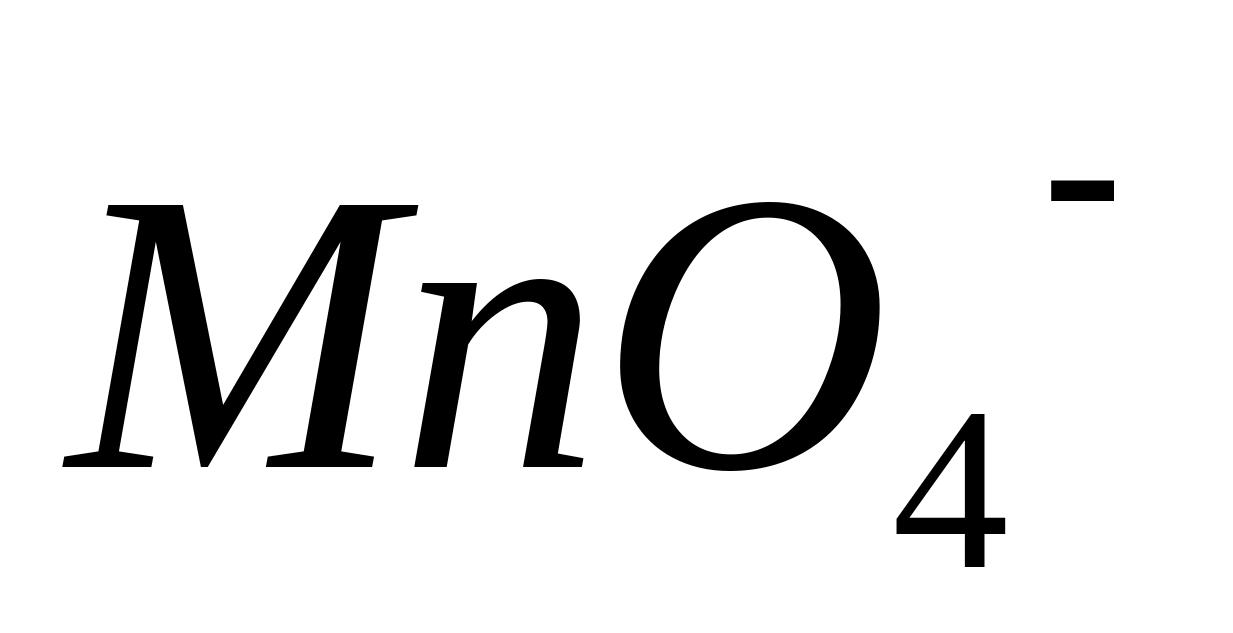 ), painted in violet-red color:
), painted in violet-red color:
or in ionic form:
In the presence of reducing agents, including 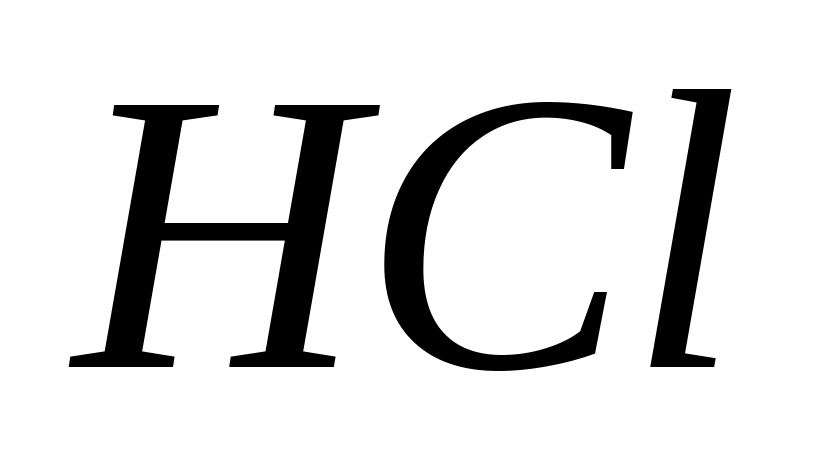 , oxidizing agents are reduced and . Therefore, solutions should not be acidified with hydrochloric acid.
, oxidizing agents are reduced and . Therefore, solutions should not be acidified with hydrochloric acid.
Carry out the oxidation of -ions to -ions. To do this, place 1-2 drops of a solution of any manganese salt (nitrate or sulfate, but not chloride !), add 5 drops of diluted (1:1) nitric acid, add a small amount of oxidizing agent (lead dioxide) and heat the mixture to a boil. Pour 1-2 ml of distilled water into the test tube, without stirring, the contents of the test tube, and let the mixture stand for a while. A crimson-red color appears, caused by the resulting permanganic acid. Since it may contain manganese compounds as an impurity, it is recommended to perform a blank experiment, observing the same conditions, but without adding the test solution to the test tube. In the absence of impurities, color does not appear.
The described oxidation reaction to permanganic acid is a very sensitive reaction.
Reaction conditions
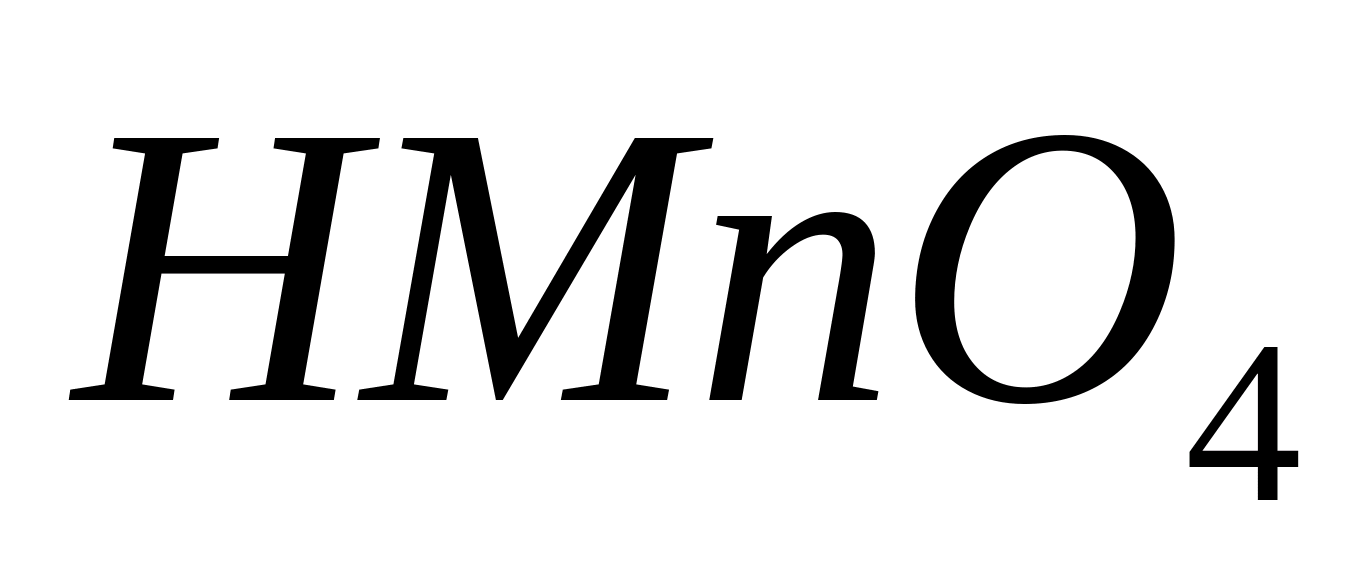
Qualitative analysis of anions
Cl-
Reaction with silver nitrate
To 1-2 ml of sodium or potassium chloride solution, add a few drops of nitric acid and solution  . In the presence of Cl - ions, a white curdled precipitate of AgCl precipitates:
. In the presence of Cl - ions, a white curdled precipitate of AgCl precipitates:
In the light the sediment darkens. To make sure that the resulting precipitate actually contains AgCl, since other ions also give similar precipitates, rinse the precipitate with distilled water and centrifuge. Drain the water. Add ammonia solution to the resulting precipitate. In this case, AgCl dissolves, forming a complex cation 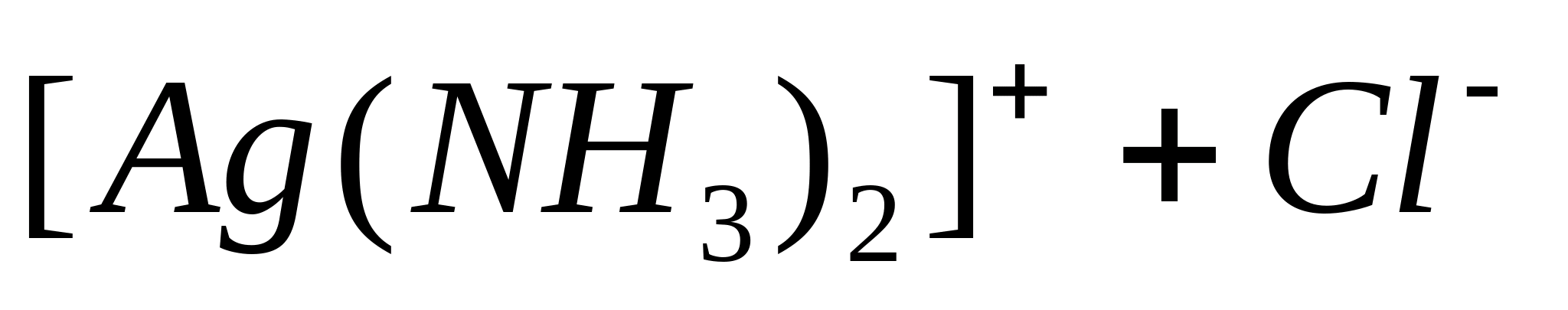 .
.
To the solution of the complex compound, add a solution of dilute  . The complex ion is destroyed and AgCl precipitates again. The appearance of a precipitate serves as evidence of the presence of Cl - ions in the analyzed substance. The described reactions proceed according to the following equations:
. The complex ion is destroyed and AgCl precipitates again. The appearance of a precipitate serves as evidence of the presence of Cl - ions in the analyzed substance. The described reactions proceed according to the following equations:

Oxidation reaction
 -ions to free chlorine
-ions to free chlorine
Place 5 drops of a solution containing -ions in a test tube, add 0.5 ml
concentrated solution  , 5 drops concentrated and heat (under traction!). In this case, partial or complete discoloration of the solution and the release of chlorine gas are observed, which is opened using starch iodide paper (blue color).
, 5 drops concentrated and heat (under traction!). In this case, partial or complete discoloration of the solution and the release of chlorine gas are observed, which is opened using starch iodide paper (blue color).
The reaction proceeds according to the equation:
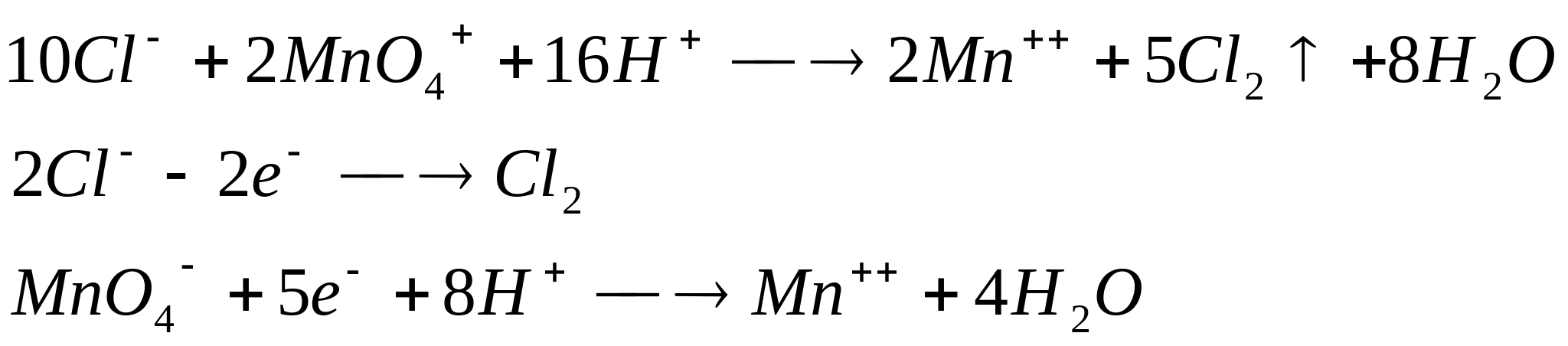
To detect the released C1 2, bring a wet
iodine starch paper. In the presence of chlorine, a blue color appears due to the release of elemental iodine:
Manganites, manganates, permanganates, manganese and lead dioxide, chromic anhydride, hypochlorous, hypochlorous and nitric acids, etc. have an oxidizing effect.
Reaction conditions.
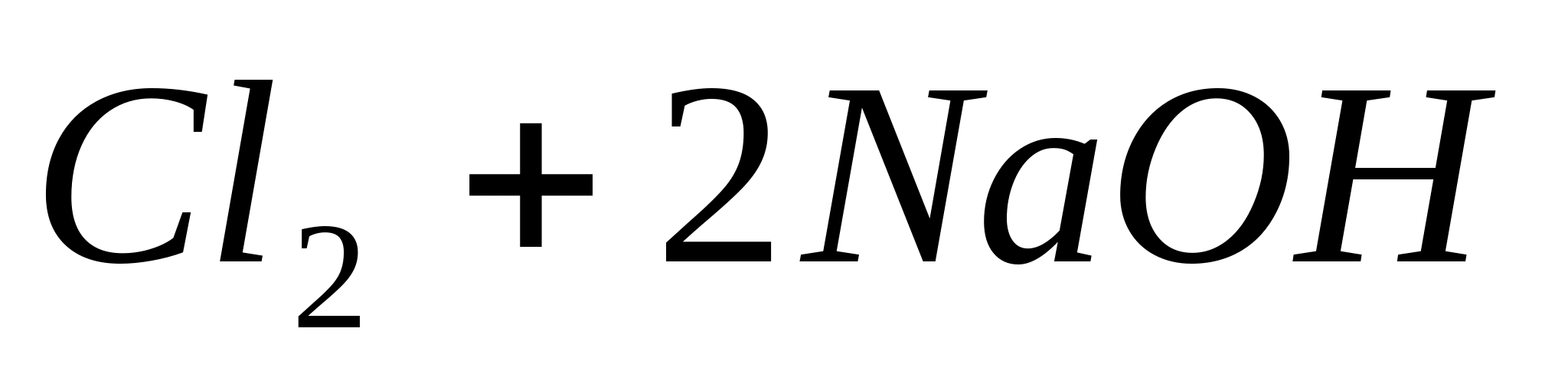
or in ionic form:
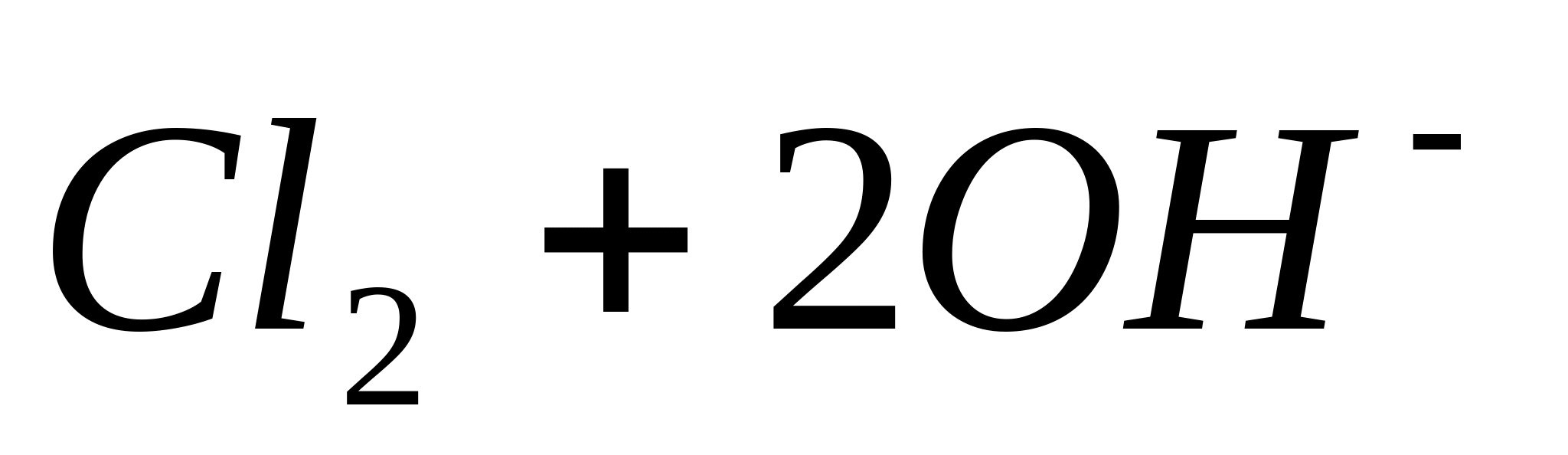
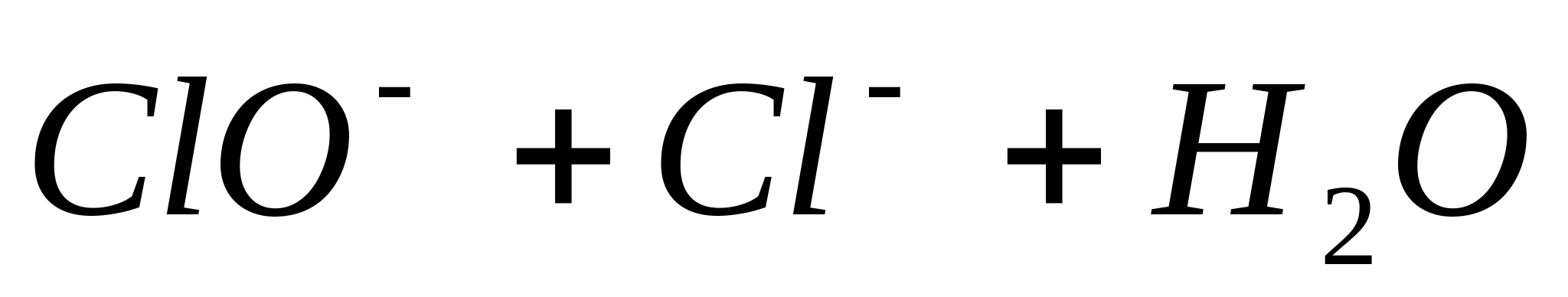
Br-
Reaction with silver nitrate
Add a few drops of nitric acid and solution to 1-2 ml of sodium or potassium bromide solution. In the presence 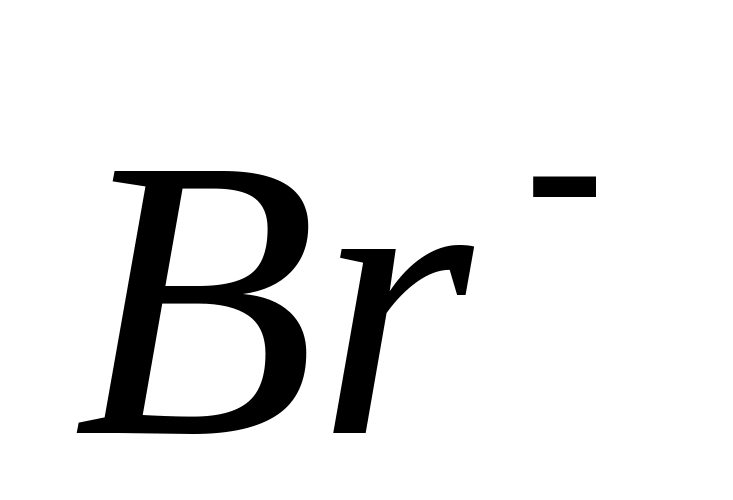 -ions, a yellowish cheesy precipitate forms AgBr. Check its solubility in sodium thiosulfate solution
-ions, a yellowish cheesy precipitate forms AgBr. Check its solubility in sodium thiosulfate solution 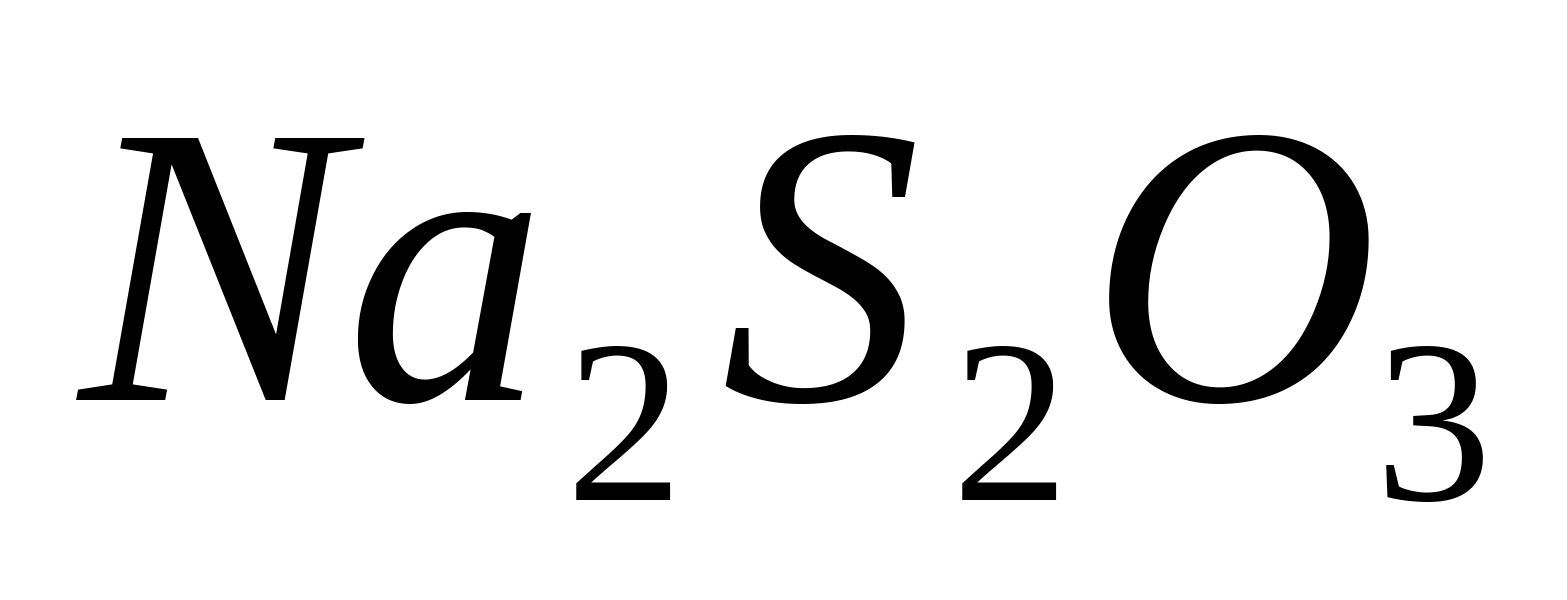 , in ammonia solution and in ammonium carbonate solution
, in ammonia solution and in ammonium carbonate solution  .
.
Oxidation reaction -ions chlorine water until free bromine
Place 5 drops of KBr solution in a test tube, 1-2 drops of diluted
0.5 ml of benzene and 2-3 drops of chlorine water. Shake the test tube. In the presence of -ions, benzene turns yellow-brown.
The reaction is applicable for the detection of -ions in the presence of - and 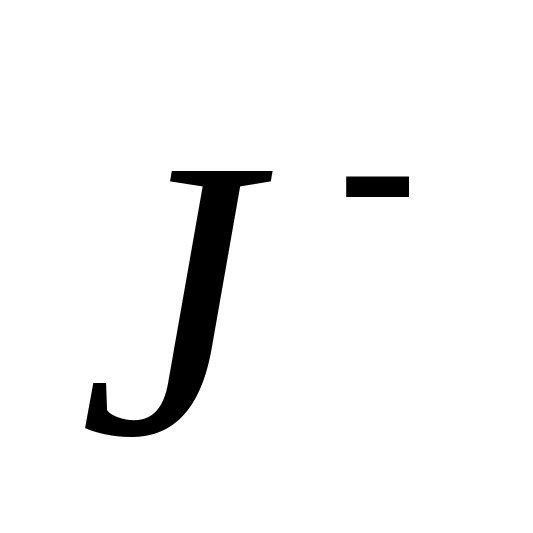 -ions.
-ions.
Reaction conditions.

J-
Reaction with silver nitrate.
Ions (as opposed to and -ions) with silver ions form a yellow cheesy precipitate, soluble only in solutions of potassium cyanide and.
Add a few drops of nitric acid and solution to 1-2 ml of sodium or potassium iodide solution. Check the solubility of the resulting precipitate in the solution.
Oxidation reaction - ions with chlorine water to free iodine
The reaction is carried out similarly to the oxidation of bromides with chlorine water. Place 5 drops of potassium iodide solution KJ, 1-2 drops of dilute sulfuric acid, 0.5 ml in a test tube
benzene and 1-2 drops of chlorine water. Shake the contents of the test tube. In the presence of -ions, the benzene layer turns red-violet:
With an excess of Cl 2, free iodine is not released and the benzene layer does not become colored:
All oxidizing agents used for the oxidation of HCl and HBr can also be used as oxidizing agents.
Reaction conditions.
Oxidation reaction -ions potassium permanganate
Place 3-5 drops of the test solution containing -ions in a test tube, acidify the solution with a few drops of diluted solution and add 1-2 drops of the solution to it.
In the presence of -ions, the solution becomes discolored in the cold and releases iodine. Moderate heating promotes the reaction:
Reaction conditions.
As soon as a red color appears, stop adding the solution and
excess is reduced with 1-2 drops of hydrogen peroxide. Excess hydrogen peroxide is decomposed by boiling the solution.
Iodate can be easily detected by adding potassium iodide to the resulting solution. In this case, iodine is released in greater quantities than during oxidation directly with permanganate:
N0 3 -
Reaction of nitrate reduction to ammonia with zinc or aluminum
Place 5 drops of potassium or sodium nitrate solution in a test tube, add 0.5 ml of NaOH or KOH solution to it and then add 25-50 mg of zinc dust or aluminum powder. To speed up the reaction, heat the mixture on a gas burner.
Zinc dust (or aluminum powder) in alkaline solutions reduces nitrates to ammonia:
The ammonia released during this process is detected as described previously.
Interaction with diphenylamine
Place 3 drops of diphenylamine solution on a glass slide  in sulfuric acid and 2 drops of sodium nitrate solution. In the presence
in sulfuric acid and 2 drops of sodium nitrate solution. In the presence  -ion appears dark blue coloring, caused by the oxidation products of diphenylamine with nitric acid.
-ion appears dark blue coloring, caused by the oxidation products of diphenylamine with nitric acid.
SO 3 --
Sulfurous acid reduction reaction
Place 3-5 drops of a solution of sulfurous acid salt (for example,  ), 3-5 drops of freshly prepared concentrated hydrochloric solution
), 3-5 drops of freshly prepared concentrated hydrochloric solution 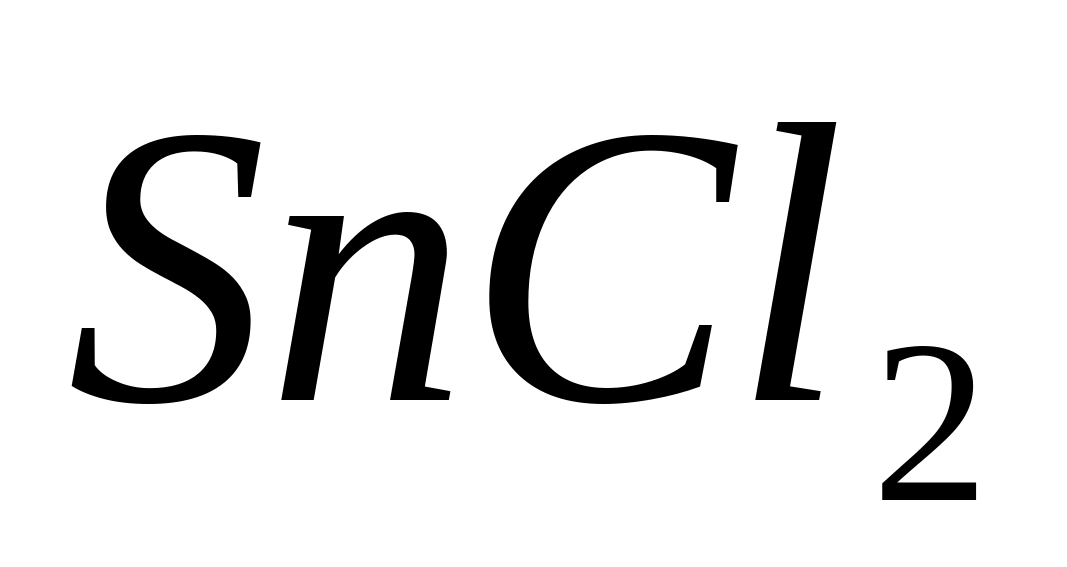 and heat the contents of the test tube. Wherein
and heat the contents of the test tube. Wherein
Place 1 drop of solution on a piece of filter paper  and 1 drop of sodium rhodizonate or rhodizonic acid solution. This produces a red spot of barium rhodizonate. Moisten the red spot with 1-2 drops of sodium sulfate solution. In the presence of sulfates, the color of barium rhodizonate immediately disappears. Barium ions with sodium rhodizonate or rhodizonic acid give a red-brown precipitate that is not decomposed by dilute HC1. Barium rhodizonate is instantly discolored by sulfates and sulfuric acid due to the formation of insoluble barium sulfate. The reaction in question is specific and is used only for the detection of sulfates.
and 1 drop of sodium rhodizonate or rhodizonic acid solution. This produces a red spot of barium rhodizonate. Moisten the red spot with 1-2 drops of sodium sulfate solution. In the presence of sulfates, the color of barium rhodizonate immediately disappears. Barium ions with sodium rhodizonate or rhodizonic acid give a red-brown precipitate that is not decomposed by dilute HC1. Barium rhodizonate is instantly discolored by sulfates and sulfuric acid due to the formation of insoluble barium sulfate. The reaction in question is specific and is used only for the detection of sulfates.
CO 3 --
R 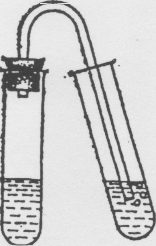 reaction of formation of carbon dioxide (carbon dioxide)
reaction of formation of carbon dioxide (carbon dioxide)
Place 1 ml of sodium carbonate solution Na 2 CO 3 in a test tube, add 2 M HC1 solution to it and quickly close the test tube with a stopper into which the outlet tube is inserted. Place the other end of this tube into a test tube with lime water (Fig.).
Carbon dioxide, passing through a solution of Ca(OH) 2, forms a white precipitate or cloudiness of CaCO 3.
Write the equation for the reaction in molecular and ionic forms.
RO 4 ---
Reaction with ammonium molybdate
Pour 1 ml of sodium phosphate solution into a test tube 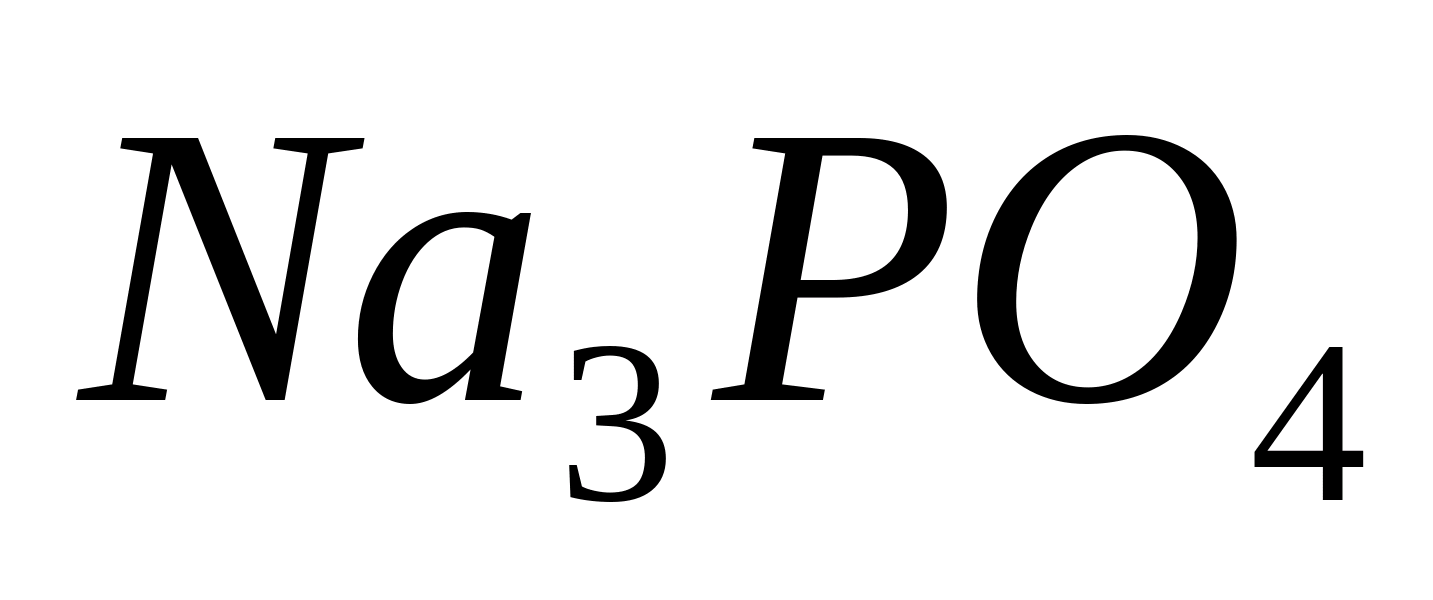 or potassium, add a few drops of 6M HNO 3 and a little solid salt - ammonium molybdate (NH 4) 2 MoO 4.
or potassium, add a few drops of 6M HNO 3 and a little solid salt - ammonium molybdate (NH 4) 2 MoO 4.
Heat the contents of the test tube. A yellow precipitate of ammonium phosphomolybdate appears. 4 3- P.O. 4 + + 3NH 4 2- + 12 MoO + + 24 H 4 ) 3 Heat the contents of the test tube. A yellow precipitate of ammonium phosphomolybdate appears. 4 = (NH 3 ∙12 MoO 2 ∙2H 2 O↓ + 10 H
O
The precipitate easily dissolves in an aqueous ammonia solution.
Control task.
Salt given. Determine which cation and anion are included in its composition.
Salt analysis
1. Preliminary tests
a) Flame coloring.
Na+ - yellow
Ca 2+ - brick red
K+ - purple
Ba 2+ - yellow-green
Cu 2+ - green.
c) Checking the pH of aqueous solutions.
If pH > 7, then the salt contains a strong base cation (alkali or alkaline earth metal)
If pH = 7, then it is a salt formed by a strong base and a strong acid. G) – Effect of dilute sulfuric acid – only carbonate ions CO 3 2
decompose by dilute acids releasing CO 2 . e) Effect of concentrated sulfuric acid
– decomposes Cl -, Br –, J -, NO 3 -, MnO 4 - releasing Cl 2, HCl, HBr, Br 2, J 2, NO 2, O 2, etc.
f) Detection of some cations. 4 +
N.H.
Add sodium hydroxide solution to the sample and heat. If there is an ammonium ion in the salt, the smell of ammonia appears, and a white coating of ammonium chloride appears on a stick moistened with a solution of concentrated hydrochloric acid.
Fe 3+
Add K4 solution to the sample. A Prussian blue precipitate forms.
Add a few drops of KSNS to the sample. A red color appears.
Fe 2+ Add K3 solution to the sample. The appearance of Turnbull's blue is noted.
2. Progress of analysis .
1. First, cations are determined in the sample.
2. At the second stage of analysis, anions are determined in the sample. Before this, preliminary tests are carried out for the content of Cl - and SO 4 -2 ions. To do this, solutions of AgNO 3 and Ba(NO 3) 2 are added to individual portions of the sample. The precipitation of a curdled sediment of AgCl and crystalline BaSO4 indicates the presence of these ions in the sample.
DocumentTest No. 1 Qualitative analysis. Heterogeneous equilibria SAMPLE SOLUTION Qualitative analysis Group and quality reactions to cations and anions necessary...
Analysis of hormones, antigens, antibodies and vitamins (2)
AnalysisTenge 590 tenge 179. Eosinophilic cationic protein (ECP) 5 p.d. - ... w.d. - 1500 tenge 52. Hepatitis B virus ( qualitative analysis) /Real-time/ (sensitivity 5 IU... 3-5 w.d.-5000 tenge 54. Hepatitis D virus ( qualitative analysis) /Real-time/ blood with EDTA 1-2 ...
Work program of the discipline “analytical chemistry” Profession: “Laboratory assistant-ecologist”
Working programmFor coloring complexes. 1h TOPIC: Qualitative analysis cations and anions 1. Interaction of barium ions... (1). Topic 6 Qualitative analysis cations and anions. Hydrogen sulfide classification cations. First analytical group cations (cations sodium groups...
Dyldina Yulia
The flame can have a different color, it all depends only on the metal salt that is added to it.
Download:
Preview:
MAOU secondary school No. 40
Subject
Flame coloring as one of the methods of analytical chemistry.
Dyldina Yudiya,
9th grade, MAOU secondary school No. 40
Supervisor:
Gurkina Svetlana Mikhailovna,
Biology and chemistry teacher.
Perm, 2015
- Introduction.
- Chapter 1 Analytical chemistry.
- Chapter 2 Methods of analytical chemistry.
- Chapter 3 Flame coloring reactions.
- Conclusion.
Introduction.
From early childhood I was fascinated by the work of chemical scientists. They seemed like wizards who, having learned some hidden laws of nature, created the unknown. In the hands of these wizards, substances changed color, caught fire, heated or cooled, and exploded. When I came to chemistry lessons, the curtain began to rise, and I began to understand how things happen. chemical processes. The chemistry course I took was not enough for me, so I decided to work on a project. I wanted the topic I was working on to be meaningful, help me better prepare for the chemistry exam, and satisfy my craving for beautiful and vivid reactions.
Flame coloration by metal ions in different colors We study it in chemistry lessons when we cover alkali metals. When I became interested in this topic, it turned out that in in this case, it is not fully disclosed. I decided to study it in more detail.
Target: With the help of this work I want to learn how to determine the qualitative composition of some salts.
Tasks:
- Get acquainted with analytical chemistry.
- Study the methods of analytical chemistry and choose the most suitable one for my work.
- Using an experiment, determine which metal is included in the salt.
Chapter 1.
Analytical chemistry.
Analytical chemistry -branch of chemistry studying chemical composition and partly the structure of substances.
The purpose of this science is to determine the chemical elements or groups of elements that make up substances.
The subject of its study is the improvement of existing and development of new methods of analysis, the search for their possibilities practical application, study of the theoretical foundations of analytical methods.
Depending on the purpose of the methods, a distinction is made between qualitative and quantitative analysis.
- Qualitative analysis is a set of chemical, physicochemical and physical methods used to detect elements, radicals and compounds that are part of the analyzed substance or mixture of substances. IN qualitative analysis you can use easily executable, characteristic chemical reactions in which the appearance or disappearance of color, the release or dissolution of a precipitate, the formation of gas, etc. are observed. Such reactions are called qualitative and with the help of them you can easily check the composition of the substance.
Qualitative analysis is most often carried out in aqueous solutions. It is based on ionic reactions and allows you to detect cations or anions of substances that are contained there. Robert Boyle is considered the founder of this analysis. He introduced this idea of chemical elements as non-decomposable basic parts of complex substances, after which he systematized all known in his time qualitative reactions.
- Quantitative analysis is a set of chemical, physicochemical and physical methods for determining the ratio of components included in the composition
analyte. From the results of this, equilibrium constants, solubility products, molecular and atomic masses can be determined. Such an analysis is more difficult to perform, since it requires a careful and more painstaking approach; otherwise, the results may produce high errors and the work will be reduced to zero.
Quantitative analysis is usually preceded by qualitative analysis.
Chapter 2.
Methods of chemical analysis.
Chemical analysis methods are divided into 3 groups.
- Chemical methodsbased on chemical reactions.
In this case, only those reactions that are accompanied by a visible external effect can be used for analysis, for example, a change in the color of the solution, the release of gases, the precipitation or dissolution of precipitation, etc. These external effects will serve in this case as analytical signals. The chemical changes that occur are called analytical reactions, and the substances that cause these reactions are called chemical reagents.
All chemical methods divided into two groups:
- The reaction is carried out in solution, the so-called “wet route”.
- A method of performing analysis on solids without the use of solvents is called the “dry route.” It is divided into pyrochemical analysis and trituration analysis. Atpyrochemical analysis andThe substance being tested is heated in the flame of a gas burner. In this case, volatile salts (chlorides, nitrates, carbonates) of a number of metals give the flame a certain color. Another method of pyrotechnic analysis is the production of colored pearls (glass). To obtain pearls, salts and metal oxides are fused with sodium tetraborate (Na2 B4O7 "10H2O) or sodium ammonium hydrogen phosphate (NaNH4HP04 4H20) and the color of the resulting glasses (pearls) is observed.
- Rubbing method was proposed in 1898 by F. M. Flavitsky. The solid test substance is ground with a solid reagent, and the external effect is observed. For example, cobalt salts with ammonium thiocyanate can give a blue color.
- When analyzed by physical methodsstudy physical properties substances using instruments without resorting to chemical reactions. TO physical methods This includes spectral analysis, luminescence, X-ray diffraction and other methods of analysis.
- Using physicochemical methodsstudy the physical phenomena that occur in chemical reactions. For example, with the colorimetric method, the color intensity is measured depending on the concentration of the substance; in the conductometric analysis, the change in the electrical conductivity of solutions is measured.
Chapter 3.
Laboratory work.
Flame color reactions.
Target: To study the coloring of an alcohol lamp flame by metal ions.
In my work, I decided to use the method of pyrotechnic analysis of flame coloring with metal ions.
Test substances:metal salts (sodium fluoride, lithium chloride, copper sulfate, barium chloride, calcium chloride, strontium sulfate, magnesium chloride, lead sulfate).
Equipment: porcelain cups, ethyl alcohol, glass rod, concentrated hydrochloric acid.
To carry out the work, I made a solution of salt in ethyl alcohol and then set it on fire. I carried out my experiment several times, on last stage The best samples were selected, and we made a video about it.
Conclusions:
Volatile salts of many metals color the flame in various colors characteristic of these metals. The color depends on the hot vapors of free metals, which are obtained as a result of the thermal decomposition of salts when they are introduced into the burner flame. In my case, these salts included sodium fluoride and lithium chloride; they gave bright, saturated colors.
Conclusion.
Chemical analysis is used by humans in many areas, but in chemistry lessons we become acquainted with only a small area of this complex science. The techniques used in pyrochemical analysis are used in qualitative analysis as a preliminary test when analyzing a mixture of dry substances or as screening reactions. In qualitative analysis, “dry” reactions play only an auxiliary role; they are usually used as primary tests and verification reactions.
In addition, these reactions are used by humans in other industries, for example, in fireworks. As we know, fireworks are decorative lights of various colors and shapes, obtained by burning pyrotechnic compositions. So, pyrotechnicians add a variety of flammable substances to the composition of fireworks, among which non-metallic elements (silicon, boron, sulfur) are widely represented. During the oxidation of boron and silicon, a large amount of energy is released, but gas products are not formed, so these substances are used to make delayed fuses (to ignite other compounds at a certain time). Many mixtures include organic carbonaceous materials. For example, charcoal(used in black powder, fireworks shells) or sugar (smoke grenades). Chemically active metals are used (aluminum, titanium, magnesium), whose combustion during high temperature gives a bright light. This property was used to launch fireworks.
In the process of work, I realized how difficult and important it is to work with substances; not everything was successful fully, as I would like. As a rule, chemistry lessons lack practical work, thanks to which theoretical skills are developed. The project helped me develop this skill. In addition, it was with great pleasure that I introduced my classmates to the results of my work. This helped them consolidate their theoretical knowledge.
♣ Flame coloring with metal salts
Salts of some metal elements (* which ones?) when introduced into the flame, they color it. This property can be used in qualitative analysis to detect cations of these elements in the sample being studied.
To carry out the experiment, a nichrome wire is required. It should be washed with conc. HCl and ignite in a burner flame. If the flame is colored when adding the wire, repeat the HCl treatment.
Immerse the wire in the solution of the salt being tested and bring it into the flame. Note coloring. After each experiment, rinse and ignite the wire until the color of the flame disappears.
Experiments on the topic “Metals of groups I and II”
1. Flame coloring
Conduct an experiment on coloring the flame with chlorides of alkali and alkaline earth metals. * Why do they take chlorides and not other salts?
Flame coloring with salts (from left to right): lithium, sodium, potassium, rubidium, cesium, calcium, strontium, barium.



(photo of potassium flame - V.V. Zagorsky)
2. Combustion of magnesium in air
Take a piece of magnesium strip with crucible tongs and burn it over a porcelain cup. Prove what the product is. * How to do it?
3. Interaction of magnesium with water and acids
A) Pour some water into the test tube, add phenolphthalein and add a little magnesium powder. If necessary, heat the test tube. * Remember how calcium interacts with water.
B) Pour 1 ml of conc. into one test tube. HCl, and in the second - 1 ml of conc. HNO3. Place a piece of magnesium tape into each test tube. * What products are formed? How can this be proven?

Experiments on the topic “Aluminum”
1. Interaction of aluminum with acids and alkalis
Study the interaction of aluminum granules with solutions in test tubes:
|
in the cold |
when heated |
|
 |
 |
|
 |
||
 |
||
 |
||
|
conc. H2SO4 |
Observations are presented in the form of a table.
* Remember how aluminum reacts withNaOH.
2. Aluminum hydroxide
Prepare aluminum hydroxide in three test tubes by dropping 1 M ammonia solution to 1 ml of aluminum salt solution. Treat the hydroxide in the first test tube with an excess of ammonia solution, in the second with an HCl solution, and in the third with a NaOH solution. In the solution obtained in the third test tube (* what is this solution?), skip CO 2. * How and in what device to get it?

3. Hydrolysis of aluminum salts
A) Determine the pH of the aluminum chloride solution. * Explain the result using the constant of the corresponding process.
B) Add 1 M sodium carbonate solution to the aluminum chloride solution.
4. Aluminothermy(one of the experiments, to choose from, is carried out under traction, in the presence of a teacher)
A) Aluminothermic production of chromium
Place a dry homogeneous mixture of 3 g of calcium fluoride powder (* what is it for? (photo by V. Bogdanov)
B) Aluminothermic production of iron
Place a dry homogeneous mixture of 1.8 g of iron (III) oxide and 0.5 g of freshly sawn aluminum powder into a fireclay crucible (or a pound made from asbestos). Make a hole in the middle and pour 0.8 g of potassium permanganate into it. In the middle of the pile of permanganate, use an empty test tube to make another hole. Place the crucible in a sand bath so that it is completely covered in sand. Pour a little glycerin on top so that it comes into contact only with the permanganate, but not with the surface of the reaction mixture. At the end of the reaction, let the crucible cool, break it and remove the “crown” of iron.

The color of the flame is violet, bb the discovery of potassium by the color of the flame in the presence of sodium is indicated in the chapter on the latter.
Flame coloring is karma and new red.
Does not color the flame.
The coloring of the flame is due to the jump of electrons from orbits more distant from the nucleus of the corresponding atom to those closer to it. Excitation is carried out in this case due to the thermal energy of the flame.
A yellow flame indicates the presence of sodium in the substance.
The yellow color of the flame indicates the presence of sodium in salt A.
A yellow flame indicates the presence of sodium in the substance.
For example, due to the heat of the flame, sodium atoms absorb 200 8 kJ / mol of heat, while the atom is excited and electrons move to higher energy levels.
The coloring of the flame when alkali metals or their compounds are added to it is caused by electronic transitions of pre-excited atoms. During the reverse transition of electrons, radiation appears, which is perceived as the color of the flame.
Flame coloration is used as a test method to detect barium. Barium salts and their solutions color the flame yellow-green.
The coloring of the flame when alkali metals or their compounds are added to it is caused by electronic transitions of pre-excited atoms. During the reverse transition of electrons, radiation appears, which is perceived as the color of the flame.
Coloring the flame with salts of calcium, strontium and barium is carried out using a clean platinum wire, moistened with solutions of volatile salts of these metals and introduced into the flame of an alcohol lamp, one at a time.
Flame coloring with strontium and barium salts is used to obtain signal signals, or so-called sparklers.
Flame coloring ion opening 58 direct and reverse 80 ion separation 58 speed 111 ate.
Coloring a gas burner flame with metal compounds is used in qualitative analysis to discover metal cations that emit radiation in the visible region of the spectrum.
If flame coloring does not reveal the presence of sodium, then separation from magnesium is not necessary. To discover magnesium and potassium when they are present together, part of the solution is tested for Mg using Na.
To color the flame, compounds of certain metals are introduced into the compositions. At high temperatures developed during combustion of compositions, metal compounds partially or completely dissociate and, having passed into a vapor state, give off an emission spectrum. Each metal produces a spectrum of its own characteristic color.
The flame turns purple.
Flame coloring reactions do not make it possible to make such conclusions, but making them can be very important in order to choose the most suitable methods quantitative analysis of a substance.
Flame coloring reactions and the production of colored pearls are carried out with a small amount of a substance, which is introduced on a platinum or nichrome wire into the flame of a gas burner or blowpipe. Salt decomposition reactions or sublimation are carried out in test tubes made of refractory glass, in porcelain and metal cups or crucibles.
Does not produce a flame coloring reaction.
The flame coloring reaction does not produce.
The flame color reaction is a highly sensitive sodium discovery reaction. The flame coloring reaction is carried out as follows: a small crystal of sodium salt is introduced on a platinum loop into the colorless burner flame, which turns yellow.
Flame coloring reactions and the production of colored pearls are carried out with a small amount of a substance, which is introduced on a platinum or nichrome wire into the flame of a gas burner or blowpipe.
The reasons for the coloring of the flame have not yet been clarified. However, this phenomenon is not caused by the combustion of SnH4, which was previously believed to be formed during the reduction of tin compounds with zinc metal.
Flame coloring reactions and the production of colored pearls are carried out with a small amount of the substance on a platinum or nichrome wire introduced into the flame of a gas burner or blowpipe.
The reasons for the coloring of the flame have not yet been clarified. However, this phenomenon is not caused by the combustion of SnH4, which was previously believed to be formed during the reduction of tin compounds with zinc metal.
According to the color of the flame orange-red color, if the calcium compound is volatile.
By the intense yellow color of the flame, which disappears when viewed through cobalt (blue) glass.
What explains the coloring of the flame when salts of a number of metals are introduced?
When performing a flame coloring reaction, the test substance is introduced into a colorless burner flame on a loop of platinum (or nichrome) wire.
A sample is tested for flame coloring, since some elements that form volatile compounds color the flame in their characteristic color, for example, barium - yellow-green, sodium - yellow.
A sample is tested for flame coloring, since some elements that form volatile compounds color the flame in their characteristic color, for example, barium - yellow-green, sodium - yellow.
In flame color tests, it may happen that the color originating from one of the analyzed elements present in large quantities, will mask the color from other elements.
Decomposition products and chemical composition of the test substance. For the flame coloring test, it is recommended to take a wire 60 mm long and 0 3 mm in diameter. One end of this wire is bent into a loop with a diameter of 2 - 3 mm, the other end is carefully soldered into a glass rod, which serves as a handle. The wire must be well cleaned by repeated calcination in the hottest part of the non-luminous burner flame. Calcination is alternated with lowering the end of the wire into concentrated hydrochloric acid.
Thallium is discovered by flame coloring and the spectral method, as well as by the formation of characteristic crystals when exposed to monovalent thallium salts of potassium iodide, chloroplatinate, chromate 13], thiosulfate, iodobismuthite [5J, uric acid, picrolonic acid 17], by the appearance of a characteristic color upon action benzidine (or o-toluidine) on trivalent thallium salt.
What color reactions to flame coloring can be performed for group IV of elements.
Externally manifested in the form of flame coloring, the emission of light rays by heated alkali metal atoms is caused by the jump of electrons from higher to lower energy levels.